BY 123 SI Session 09/22/15 Chapter 7 What's amphipathic mean? 1
advertisement

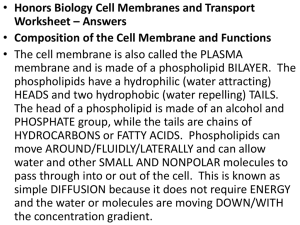
BY 123 SI Session 09/22/15 Chapter 7 What’s amphipathic mean? 1. Who proposed the sandwich model (phospholipid bilayer between two layers of hydrophilic proteins)? 2. Who proposed the fluid mosaic model? 3. What’s the fluid mosaic model? What research experiment allows us to accept this hypothesis? 4. What can we say about polarity within regions of the membrane proteins? 5. All of the following molecules are part of the cell membrane except A) lipids. B) nucleic acids. C) proteins. D) phosphate groups. E) steroids. 6. Can phospholipids move? What’s the function of cholesterol in the plasma membranes of some animals? 7. What’s the relationship between temperature and membrane lipid composition? 8. What’s the function of glycoproteins and glycolipids sticking out the cell? Know Figure 7.9 Membrane proteins and lipids are synthesized in the ER -> carbs, and proteins can be joined together and sent to the Golgi -> glycoproteins undergo further carb modification and lipids acquire carbohydrates, becoming glycolipids -> these glycoproteins, glycolipids, and secretory proteins are transported to vesicles to the plasma membrane -> release through exocytosis 9. What are transport proteins and give me two examples. 10. Give me examples when channel proteins are gated or ungated. 11. What type of change happens for carrier proteins to function properly? 12. Passive transport consists of what three terms? 13. Simple diffusion characteristics explained in class? 14. Explain isotonic, hypertonic, and hypotonic solutions in terms with water. Know Figure 7.12 15. What is facilitated diffusion? 16. The solutions in the two arms of this U-tube are separated by a membrane that is permeable to water and glucose but not to sucrose. Side A is half filled with a solution of 2 M sucrose and 1 M glucose. Side B is half filled with 1 M sucrose and 2 M glucose. Initially, the liquid levels on both sides are equal. Initially, in terms of tonicity, the solution in side A with respect to that in side B is A) hypotonic. B) plasmolyzed. C) isotonic. D) saturated. E) hypertonic. After the system reaches equilibrium, what changes are observed? A) The molarity of sucrose and glucose are equal on both sides. B) The molarity of glucose is higher in side A than in side B. C) The water level is higher in side A than in side B. D) The water level is unchanged. E) The water level is higher in side B than in side A. 17. What happens if you add unionized (distilled) water to blood? What’s hypotonic comparatively? 18. Which of the following statements correctly describes the normal tonicity conditions for typical plant and animal cells? A) The animal cell is in a hypotonic solution, and the plant cell is in an isotonic solution. B) The animal cell is in an isotonic solution, and the plant cell is in a hypertonic solution. C) The animal cell is in a hypertonic solution, and the plant cell is in an isotonic solution. D) The animal cell is in an isotonic solution, and the plant cell is in a hypotonic solution. E) The animal cell is in a hypertonic solution, and the plant cell is in a hypotonic solution. 19. What is active transport? Know Figure 7.16 20. Know the sodium-potassium pump and the proton pump (plants, fungi, and bacteria)? 21. What is cotransport? 22. 3 types of endocytosis are Figure 7.19 A cell (concentration .8 M glucose and .01 M sucrose) with a semi-permeable membrane only to water is placed in a solution with .7 M glucose, .01 M sucrose, and .2 M fructose. Where will water flow? Molarity = moles/liters A problem could give you TWO unknowns: grams, molarity, moles, and liters. They can give you grams because given grams, it’s easy to find to amount of moles! Grams = molecular weight (g/mol) X moles Moles = grams/molecular weight