Thermochemistry Review
advertisement

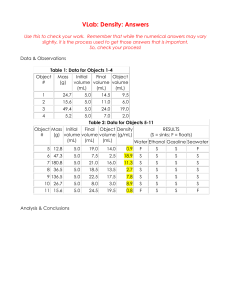
Aim: How well do I know the material from this unit? Do Now: Take a Calorimetery Worksheet and Do all Odd problems Homework: 1. 2. Castle Learning Due Tomorrow STUDY for TEST Tomorrow Flammable Ice Ice...? on Fire Answer 1. 2. 3. 4. Where is kinetic energy increasing? Where is potential energy increasing? Where is the Solid phase only present? Where is both liquid and gas present at the same time? 5. 6. 7. Where is the Heat of Fusion? Where is the Heat of Vaporization? Which one requires more energy and how do you know? 8. 9. 10. Name the 2 phase changes described in this graph and describe which line segment represents each specific change? Are these 2 phase changes exothermic or endothermic? Which point has the lowest kinetic energy? Cold packs are used to treat minor injuries. Some cold packs contain NH4NO3(s) and a small packet of water at room temperature before activation. To activate this type of cold pack, the small packet must be broken to mix the water and NH4NO3(s). The temperature of this mixture decreases to approximately 2°C and remains at this temperature for 10 to 15 minutes. 13. State the direction of heat flow that occurs when the activated cold pack is applied to the body. Which change results in a release of energy? 1. the melting of H2O(s) 2. the boiling of water () 3. the evaporation of H2O() 4. the condensation of H2O(g) Answer =4 If two systems at different temperatures have contact with each other, heat will flow from the system at 1. 20°C to 2. 30°C to 3. 40°C to 4. 50°C to a a a a Answer = 3 system system system system at 303 K at 313 K at 293 K at 333 K During which change is energy absorbed? 1. the melting of ice 2. the cooling of ice 3. the freezing of water 4. the condensation of water Answer =1 Two pure water samples held in separate containers at one atmosphere pressure must have molecules possessing the same average kinetic energy if the samples have the same 1. volume 2. temperature 3. mass 4. density Answer =2 The amount of heat needed to change 1 gram of a solid to 1 gram of a liquid at constant temperature is called the heat of 1. vaporization 2. sublimation 3. fusion 4. fission Answer =3 The average kinetic energy of water molecules is greatest in which of these samples? 1. 10 g of water at 35°C 2. 10 g of water at 55°C 3. 100 g of water at 25°C 4. 100 g of water at 45°C The temperature of a piece of Metal with a mass of 95.4g increases from 25.0°C to 48.0°C as the metal absorbs 849 J of heat. What is the specific heat of Metal X? 0.387 J/g°C How much energy is required to vaporize 10.g of water at its boiling point? q = 22600j or 22.6kJ How much energy is released when 20. g of water is frozen at 0ºC? q = 6680j or 6.68kJ Calculate the amount of heat needed to increase the temperature of 250g of water from 20oC to 46oC. Write your answer in kiloJoules. q = 37,620J or 38kJ The temperature of 15 grams of water increased 3.0°C. How much heat was absorbed by the water? 216 J of energy is required to raise the temperature of aluminum from 15o to 35oC. Calculate the mass of aluminum. (Specific Heat Capacity of aluminum is 0.90) m= 12g How much energy is required to melt 10.g of ice at its melting point? q = 3340J or 3.34kJ The initial temperature of 150g of ethanol was 22oC. What will be the final temperature of the ethanol if 3240 J was needed to raise the temperature of the ethanol? (Specific heat capacity of ethanol is 2.44) T = 8.85oC Tfinal= 22oC +8.85oC= 30.9oC Based on data collected during a laboratory investigation, a student determined an experimental value of 322 joules per gram for the heat of fusion of H2O. Calculate the student’s percent error. Answer: 3.6% How much energy is released when 20. g of steam is condensed at 100ºC? q = 45200j or 45.2kJ Which measurement has the greatest number of significant figures? 1. 6.060 mg 2. 60.6 mg 3. 606 mg 4. 60600 mg Expressed to the correct number of significant figures, the sum of two masses is 445.2 grams. Which two masses produce this answer? 1. 210.10 g + 235.100 g 2. 210.100 g + 235.10 g 3. 210.1 g + 235.1 g 4. 210.10 g + 235.10 g When sodium chloride is dissolved in water, the resulting solution is classified as a 1. heterogeneous compound 2. homogeneous compound 3. heterogeneous mixture 4. homogeneous mixture During fractional distillation, hydrocarbons are separated according to their 1. boiling points 2. melting points 3. triple points 4. saturation points An example of a physical property of an element is the element’s ability to 1. react with an acid 2. react with oxygen 3. form a compound with chlorine 4. form an aqueous solution Which substance cannot be decomposed into simpler substances? 1. ammonia 2. aluminum 3. methane 4. methanol A sample of water is heated from a liquid at 40°C to a gas at 110°C. The graph of the heating curve is shown below. 11. For section QR of the graph, state what is happening to the water molecules as heat is added. 12. For section RS of the graph, state what is happening to the water molecules as heat is added.