CH 235-2F Worksheet 6-Exam 2 September 29, 2014
advertisement

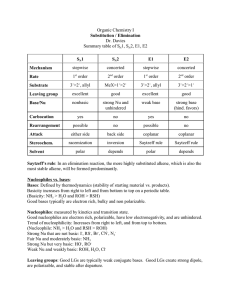
Page 1 of 3 CH 235-2F Worksheet 6-Exam 2 September 29, 2014 Short Answer: 1. What are the three definitions for acids/bases? Give an example of each. Which one is most useful for organic chemistry? 2. Fill in the table below regarding factors that affect acidity/basicity. Factor s character Arrange the following in order of most s character to least s character: sp3, sp2, sp Size Follow this trend when going down a __________________. Which is larger, Iodine or bromine? Electronegativty Follow this trend when going across a __________________. True or False: HF is a weaker acid than NH3. Inductive Effect This involves the pulling of electron density through _____________ How does distance effect this? ____________. Why does more s character mean a strong acid/weaker conjugate base? According to your answer in 3C, is HI a stronger acid than HBr? True or false: more electronegative atoms will result in more of an inductive effect. Page 2 of 3 Factor Resonance This trend allows for __________________ of charge. Draw the resonance structure for the conjugate base of CH3COOH General Question: Does equilibrium favor the strong acid/strong base or the weak cid/weak base? 3. What is a nucleophile? What is an electrophile? Give examples of each. How do you determine which species is a nucleophile/electrophile given a reaction? 4. For each reaction below, determine which species is the nucleophile and which species is the electrophile. H20 + NH3 CH3COOH + H2O HPO42- + NH4+ NH4+ + OHCH3COO- + H3O+ NH3 + H2PO4- Matching A. B. C. D. E. F. G. H. Low pKa High pKa Low pKb High pKb High Ka Low Ka High Kb Low Kb _________1. Strong Acid _________2. Strong Base _________3. Weak Acid _________4. Weak Base Page 3 of 3 5. Arrange the following in order of increasing acidity: HS, HF, HCl, HSe What trend did you use? 6. Arrange the following from weakest to strongest conjugate base (I have shown the acid): Multiple Choice 1. Which of the following statements is true? A. HCl is a stronger acid than HI. B. A molecule that can delocalize charge due to resonance is a more stable conjugate base than one with no resonance structures. C. A carbon with spy hybridization has more s character than a carbon with sp hybridization. D. HF is a weaker acid than H2O. E. Both B and D. 2. Which of the hydrogens indicated below is most likely to be removed in an acid/base reaction? A. B. C. D. Green Arrow Blue Arrow Yellow Arrow Red Arrow