Chem Names and Formulas
advertisement

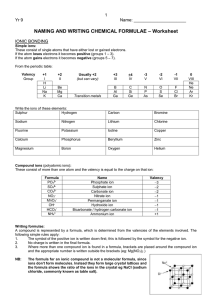
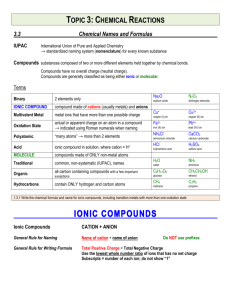
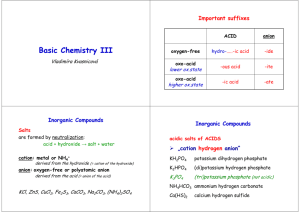
Chapter 2 Chemical Names and Formulas Naming Ions IE X + energy Atom • Cation • Positive Ion • Metal • Loses Electrons Cation t looks like a + X+ + eIon Cation Naming Ions EA X + eAtom X- + energy Ion Anion • Anion • Negative Ion • Nonmetal • Gains Electrons Anion n in middle stands for negative Monoatomic Ion Monatomic Ion – Ion that consists of a single atom • Cation • Positive Ion • Metal • Loses Electrons • Anion • Negative Ion • Nonmetal • Gain Electrons Rules for Ion Names/Symbols: Cation Metals = use element name = use symbol with superscript charge = superscript is written in air Alkali Metals Transition Metals Hydrogen H+1 Silver Ag+1 Lithium Li+1 Gold Au+1 Sodium Na+1 Potassium K+1 Rules for Ion Names/Symbols: Cation Metals = use element name = use symbol with superscript charge = superscript is written in air Alkaline Earth Metals Transition Metals Magnesium Mg+2 Cobalt Co+2 Calcium Ca+2 Nickel Ni+2 Strontium Sr+2 Zinc Zn+2 Barium Ba+2 Rules for Ion Names/Symbols: Cation Some metals have multiple charges. Stock System uses Roman numerals. Roman numerals = charge!! 1 I 6 VI 2 II 7 VII 3 III 8 VIII 4 IV 9 IX 5 V 10 X . Rules for Ion Names/Symbols: Cation Copper (I) Copper (II) Iron (II) Iron (III) Lead (II) Lead (IV) Cu+1 Cu+2 Fe+2 Fe+3 Pb+2 Pb+4 Tin (II) Sn+2 Tin (IV) Sn+4 Manganese (II) Mn+2 Manganese (IV) Mn+4 Rules for Ion Names/Symbols: Anion Nonmetals = use element name with ‘ide’ = use symbol with superscript charge = superscript is written in air Halogens Fluorine Fluoride F-1 Chlorine Chloride Cl-1 Bromine Bromide Br-1 Iodine Iodide I-1 Rules for Ion Names/Symbols: Anion Nonmetals = use element name with ‘ide’ = use symbol with superscript charge = superscript is written in air Oxygen Family Nitrogen Family Oxygen Oxide O-2 Nitrogen Nitride N-3 Sulfur Sulfide S-2 Phosphorus Carbon Family Phosphide P-3 Carbon Carbide C-4 Rules for Ion Names/Symbols: Cation or Anion ? Multiples can be cation or anion. It depends on what other ion is around it. Cation Anion Carbon C+4 Carbide C-4 Nitrogen N+5 Nitride N-3 Phosphorus P+5 Phosphide P-3 Binary Compounds Composed of 2 elements • Can be either ionic or molecular Binary Ionic Compounds Consist of Ions • Metallic Ions bonded to Nonmetal ions • or Cations bonded to Anions Writing Names for Binary Ionic Compounds Steps 1. Use ION names. 2. Write the CATION name. 3. Then write the ANION name. For example, NaCl becomes Sodium Chloride MgBr2 becomes Magnesium Bromide CuO becomes Copper (II) Oxide Writing Formulas for Binary Ionic Compounds Steps 1. USE the IONS. 2. Write the symbol for the CATION. 3. Then write the symbol for the ANION. 4. Balance the charges to zero. The subscripts denotes the number of ions needed to balance the charges to zero. Example (Level 1) Sodium Chloride Na+1 Cl-1 NaCl Example (Level 1) Magnesium Oxide Mg+2 O-2 MgO Example (Level 1) Aluminum Nitride Al+3 N-3 AlN Practice 1. 2. 3. 4. 5. 6. 7. Potassium Iodide Calcium Sulfide Aluminum Phosphide Gold Fluoride Barium Oxide Sodium Bromide Strontium Sulfide Example (Level 2) Lithium Oxide Li+1 Li+1 O-2 Li2O Example (Level 2) Calcium Fluoride Ca+2 F-1 F-1 CaF2 Example (Level 2) Aluminum Iodide Al+3 I-1 I-1 I-1 AlI3 Practice 1. 2. 3. 4. 5. 6. 7. Potassium Oxide Strontium Bromide Aluminum Chloride Gold Sulfide Silver Nitride Barium Fluoride Calcium Iodide Example (Level 3) Aluminum Sulfide Al+3 Al+3 S-2 S-2 S-2 Al2S3 Example (Level 3) Tin (IV) Nitride Sn+4 Sn+4 Sn+4 N-3 N-3 N-3 N-3 Sn3N4 Polyatomic Ions Polyatomic Ions – Ions composed of more than one atom • Most end in ite or ate Polyatomic Ions Ammonium NH4+1 Cyanide CN-1 Hydroxide OH-1 Nitrite NO2-1 Sulfite SO3-2 Phosphite PO3-3 Nitrate Carbonate Sulfate Phosphate NO3-1 CO3-2 SO4-2 PO4-3 Example (Level 1) Ammonium Chloride NH4+1 Cl- NH4Cl Example (level 1) Barium Sulfate Ba+2 SO4-2 BaSO4 Polyatomic Ions Only When writing compounds if you need more than one polyatomic ion you must add parenthesis Example (Level 2) Ammonium Sulfate NH4+1 NH4+1 NH42SO4 SO4-2 (NH4)2SO4 Example (Level 2) Barium Hydroxide Ba+2 BaOH2 OH-1 OH-1 Ba(OH)2 Practice 1. 2. 3. 4. 5. 6. 7. Ammonium Phosphate Calcium Hydroxide Aluminum Nitrate Magnesium Sulfate Tin (IV) Carbonate Strontium Sulfate Barium Nitrate Nomenclature of Ionic Compounds Steps 1. Use the names of the ions. 2. Write the metal first followed by the nonmetal 3. The subscripts take care of themselves Example NaCl Na+1 Cl-1 Sodium Chloride Example CaCl2 Ca+2 Cl-1 Cl-1 Calcium Chloride Example CuSO4 Cu+2 SO4-2 Copper (II) Sulfate Practice 1. 2. 3. 4. 5. 6. 7. NaI (NH4)2SO4 MgBr2 Al2(CO3)3 CuO Fe2O3 Ca3P2 Formula Writing and Nomenclature Covalent (Molecular) Compounds Steps 1. Write the first nonmetal symbol followed by the second nonmetal symbol. 2. The Greek prefixes denote the subscripts. 3. Do not need to balance the charges. Greek Prefixes for Molecular Compounds 1 2 3 4 5 Mono Di Tri Tetra Penta 6 Hexa 7 Hepta 8 Octa 9 Nona 10 Deca Example Carbon Dioxide C 2 O CO2 Example Dinitrogen Monoxide 2 1 N N2O O Practice 1. 2. 3. 4. 5. 6. 7. Sulfur Dioxide Trichlorine Tetraiodide Pentaphosphorus Hexasulfide Heptaoxygen Octaselenide Nonafluorine Decanitride Dinitrogen Pentoxide Carbon Tetrachloride Naming Molecular Compounds Rules 1. Write the 1st element name. 2. Write the 2nd element name with an ending of “ide” 3. Add Greek prefixes to both names. The Greek prefix denotes the subscript. 4. Do not use a mono on the 1st element name. Example SiO2 Silicon Di Oxygen Silicon Dioxide ide Example P4F6 ide Tetra Phosphorus Hexa Fluorine Tetraphosphorus Hexafluoride Practice 1. 2. 3. 4. 5. 6. 7. CO Si2F O3Br7 IS ON4 F4H3 P7S9 Halogenated Oxyanions Name: Prefix Suffix Hypo root ite root ite root ate Per root ate Example: Chlorine Hypochlorite ClO-1 Chlorite ClO2-1 Chlorate ClO3-1 Perchlorate ClO4-1 Try Bromine and Iodine. Polyatomic Oxyacids Example: Example: Chlorine Chlorine Hypochlorite ClO-1 Hypochlorous acid Chlorite ClO2-1 Chlorous acid Chlorate ClO3-1 Chloric acid Perchlorate ClO4-1 Perchloric acid HClO HClO2 HClO3 HClO4 Binary Acids Formula: Hydrogen w Halogen Name: Hydro+Root+ic Acid HCl Hydrochloric Acid HBr Hydrobromic Acid HI Hydroiodic Acid HF Hydrofluoric Acid Alkanes Methane Ethane Propane Butane Pentane Alcohols Methanol Ethanol Propanol Butanol Pentanol Other functional groups? Alcohols Ethers Aldehydes Ketones Carboxylic acid Ester Amine Amide Formula weights/Molar Mass The sum of atomic weights for a compound (in amu) The number of grams in one mole of a substance (in g) Lewis Dot Structures For atoms For ionic compounds For molecular compounds LDS: Neutral Molecules Draw the dot structures of each atom Share one atom for each element (to make a single bond between each element) Count electrons around each atom If octets are incomplete, share more electrons between atoms LDS: Negatively Charged Molecules Repeat procedure for uncharged molecules Add one electron for each negative charged atom LDS: Positively charged molecules Same procedure as negative… except remove one electron Web Practice Site http://www.fernbank.edu/Chemistry/nom en.html