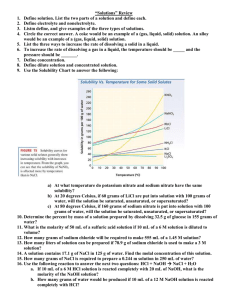
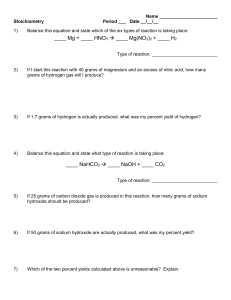
Mass to Mass Worksheet: Double Displacement Reactions
advertisement

Mass to Mass Worksheet: Double Displacement Reactions Name__________________________________________ Date__________________Per_____________________ Write the Balanced Equation for the reactants given. Show all math. Each number must have units attached and the compound formula written with the number. 1. CaCl2 + NaOH If 20g of CaCl2 is used, how many grams of sodium chloride will be produced? 2. Pb(NO3)2 + KI How many grams of lead (II) nitrate is needed to completely react 25g of potassium iodide? 3. HCl + K2SO4 How many grams sulfuric acid will be produced if 100g of hydrochloric acid is used? 4. FeO + Al2S3 If 33g of iron (II) oxide is used, how many grams of iron (II) sulfide will be produced? 5. Mg3(PO4)2 + Be(NO3)2 If 48g of beryllium nitrate is completely used up in the above reaction, how much magnesium phosphate was used? 6. Na2O + GaN If 300g of sodium oxide reacted with gallium nitride, how much sodium nitride was produced? 7. NbO + Cu3P2 If 52g of niobium (II) oxide was used, how much niobium (II) phosphide was produced? 8. Cr(C2H3O2)2 + NaCl If 45g of sodium acetate was produced, how many grams of the other product will also be produced?