VSEPR Model
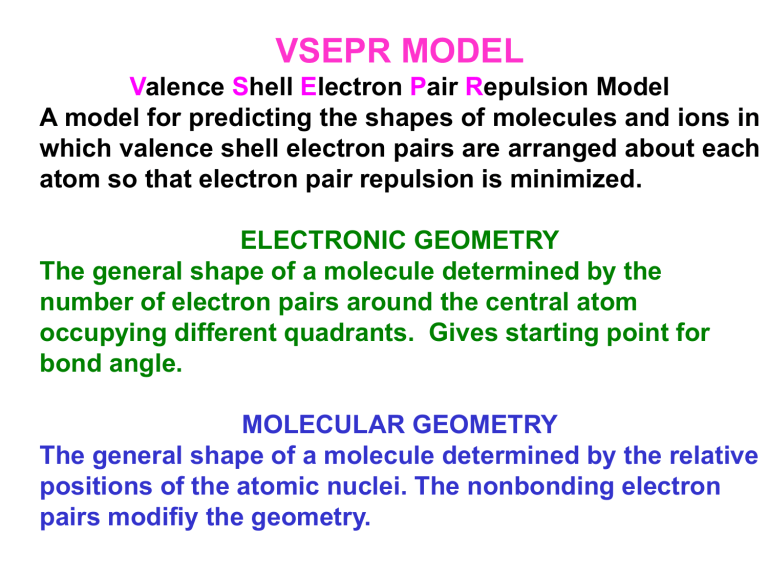
VSEPR MODEL
V alence S hell E lectron P air R epulsion Model
A model for predicting the shapes of molecules and ions in which valence shell electron pairs are arranged about each atom so that electron pair repulsion is minimized.
ELECTRONIC GEOMETRY
The general shape of a molecule determined by the number of electron pairs around the central atom occupying different quadrants. Gives starting point for bond angle.
MOLECULAR GEOMETRY
The general shape of a molecule determined by the relative positions of the atomic nuclei. The nonbonding electron pairs modifiy the geometry.
VSEPR MODEL
I . Draw the Lewis dot structure.
II.
Determine the electronic geometry by counting the number of pairs of electrons around the central atom occupying different quadrants (top, bottom, left, right).
This geometry gives the initial bond angle.
Pairs of e-
2
3
4 geometry bond angle linear 180 o trigonal planar 120 o tetrahderal 109.5
o
VSEPR MODEL
III . Next, using the electronic geometry, determine the number of bonding and nonbonding electron pairs then arrange the electron pairs as far apart as possible.
___ nonbonding pairs require more space than bonding pairs.
___ multiple bonds require more space than single bonds.
IV .
The direction in space of the bonding pairs give the molecular geometry modified by the position of the nonbonding pairs.
Fig. 13-1, p. 369
Fig. 13-2, p. 369
Fig. 13-3, p. 370
4
4
4
3
3
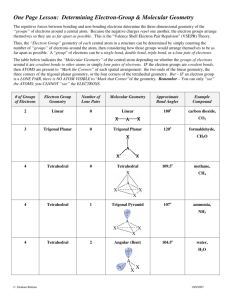
Table describing Molecular Geometry
Number of
VSPER Theory
electronic bonding nonbonding molecular e- pairs geometry e- pairs e- pairs geometry
2 linear 2 0 linear trigonal planar 3 0 trigonal planar 2 1 tetrahedral tetrahdral tetrahedral
4 0
3 1
2 2 trigonal planar bent tetrahedral trigonal pyramidal bent
Fig. 13-4, p. 370
Fig. 13-5, p. 372
Table 13-2, p. 371
p. 374
p. 374
p. 375
p. 375
p. 375
p. 376
Predict the Molecular Geometry for the following molecules.
1. H
2
O
There are 4 pairs of electrons around the central atom so the electronic geometry is tetrahedral with a bond angle of 109.5
o . Since 2 of the pairs of electrons are bonding and the other two pairs are nonbonding, the electronic geometry has been modified to a molecular geometry of
BENT.
2. CO
2
There are four pairs of electrons in two different quadrents (left & right) so the molecular geometry is
LINEAR with a bond angle of 180 o .
. . . .
: O = C = O :
Predict the Molecular Geometry for the following molecules.
3. BH
3
There are 3 pairs of electrons around the central atom so the electronic geometry is trigonal planar with a bond angle of 120 o . Since all three pairs of electrons are bonding, the electronic geometry has not been modified.
4. NH
3
There are 4 pairs of electrons around the central atom so the electronic geometry is tetrahedral with a bond angle of 109.5
o . Since 3 of the pairs of electrons are bonding and the other pair is nonbonding, the electronic geometry has been modified to a molecular geometry of
TRIGONAL PYRAMIDAL.
Practice Problems
• Draw the Lewic structure for the following, then predict both the electronic & molecular geometry. Give the approximate bond angle . (See instructor for answers) a) GeH
2 d) SO
2 g) SiF
4 b) AsF
3 e) SO
3 h) C
2
H
4 c) AlF
3 f) SO
3
2-
I) Cl
2
O
Group Study Problems
Draw the Lewic structure for the following, then predict both the electronic & molecular geometry.
Give the approximate bond angle .
a) H
2
S d) NO
2
g) CH
2
FCl b) PH
3 e) H
3
PO
4 h) C
2
H
2 c) CH
2
O f) CBr
4
I) O
3