10. Infrared spectroscopy
advertisement

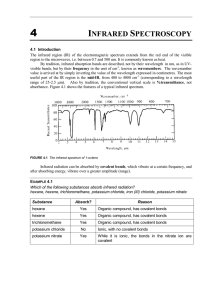
Infrared spectroscopy Keywords: infrared, radiation, absorption, spectroscopy Learning Objectives • State that absorption of infrared radiation causes covalent bonds to vibrate. • Identify absorption peaks in an infrared spectrum. • State that modern breathalysers measure ethanol levels by analysis using infrared spectroscopy. All molecules absorb infrared radiation. This absorbed energy makes bonds vibrate with either a stretching or bending motion Every bond vibrates at its own unique frequency The amount of vibration depends on: • The bond strength • The bond length • The mass of each atom involved in the bond Most bonds vibrate at a frequency between 300 and 4000cm-1, in the infrared part of the electromagnetic spectrum The absorbed energies can be displayed as an IR spectrum. By analysing this spectrum, we can determine details about a compound’s chemical structure. The spectrum indicates the presence of functional groups in the compound. A beam of infrared radiation is passed through a sample of the material under investigation. The beam contains the full range of frequencies present in the infrared region. The molecule absorbs some of these frequencies and the emerging beam is analysed to plot a graph of transmittance against frequency. Frequency is measured using wavenumbers with units cm-1 Schematic diagram of an infrared spectrometer The molecule’s spectrum has a number of troughs (called peaks). Each peak represents the absorbance of energy from infrared radiation that causes the vibration of a particular bond in the molecule under investigation Applications of infrared spectroscopy Infrared spectroscopy has many everyday uses: • It is used extensively in forensic science (eg. To analyse paint fragments from vehicles in hit-andrun offences) • Monitoring the degree of unsaturation in polymers • Quality control in perfume manufacture • Drug analysis • Testing the breath of suspected drunken drivers for ethanol