X(t) - Center for Complex Systems and Brain Sciences
advertisement
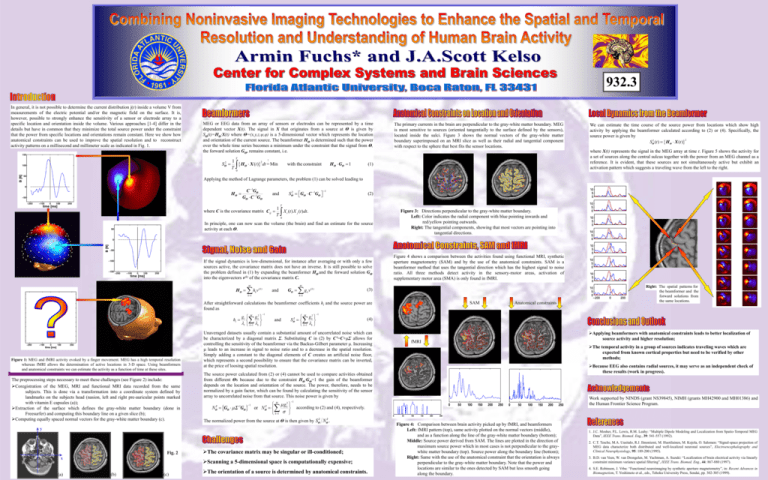
932.3 In general, it is not possible to determine the current distribution j(r) inside a volume V from measurements of the electric potential and/or the magnetic field on the surface. It is, however, possible to strongly enhance the sensitivity of a sensor or electrode array to a specific location and orientation inside the volume. Various approaches [1-4] differ in the details but have in common that they minimize the total source power under the constraint that the power from specific locations and orientations remain constant. Here we show how anatomical constraints can be used to improve the spatial resolution and to reconstruct activity patterns on a millisecond and millimeter scale as indicated in Fig. 1. MEG or EEG data from an array of sensors or electrodes can be represented by a time dependent vector X(t). The signal in X that originates from a source at Θ is given by SΘ(t)=HΘ·X(t) where Θ=(x,y,z,ψ,φ) is a 5-dimensional vector which represents the location and orientation of the current source. The beamformer HΘ is determined such that the power over the whole time series becomes a minimum under the constraint that the signal from Θ, the forward solution GΘ, remains constant, i.e. 1 S T 2 Θ T H ! X (t ) dt = Min 2 Θ H Θ GΘ 1 with the constraint The primary currents in the brain are perpendicular to the gray-white matter boundary. MEG is most sensitive to sources (oriented tangentially to the surface defined by the sensors), located inside the sulci. Figure 3 shows the normal vectors of the gray-white matter boundary superimposed on an MRI slice as well as their radial and tangential component with respect to the sphere that best fits the sensor locations. We can estimate the time course of the source power from locations which show high activity by applying the beamformer calculated according to (2) or (4). Specifically, the source power is given by 2 2 SΘ (t ) H Θ X (t ) where X(t) represents the signal in the MEG array at time t. Figure 5 shows the activity for a set of sources along the central sulcus together with the power from an MEG channel as a reference. It is evident, that these sources are not simultaneously active but exhibit an activation pattern which suggests a traveling wave from the left to the right. (1) 0 Applying the method of Lagrange parameters, the problem (1) can be solved leading to C 1GΘ HΘ GΘ C 1GΘ 1 S GΘ C GΘ 2 Θ and 1 (2) T where C is the covariance matrix Cij 1 X i (t ) X j (t )dt. T 0 In principle, one can now scan the volume (the brain) and find an estimate for the source activity at each Θ. If the signal dynamics is low-dimensional, for instance after averaging or with only a few sources active, the covariance matrix does not have an inverse. It is still possible to solve the problem defined in (1) by expanding the beamformer HΘ and the forward solution GΘ into the eigenvectors v(k) of the covariance matrix C. HΘ M h v (k ) and k GΘ k 1 Figure 3: Directions perpendicular to the gray-white matter boundary. Left: Color indicates the radial component with blue pointing inwards and red/yellow pointing outwards. Right: The tangential components, showing that most vectors are pointing into tangential directions. Figure 4 shows a comparison between the activities found using functional MRI, synthetic aperture magnetometry (SAM) and by the use of the anatomical constraints. SAM is a beamformer method that uses the tangential direction which has the highest signal to noise ratio. All three methods detect activity in the sensory-motor areas, activation of supplementary motor area (SMA) is only found in fMRI. M g v (3) (k ) k k 1 SAM After straightforward calculations the beamformer coefficients hi and the source power are found as gl g hl l k 1 k M Figure 1: MEG and fMRI activity evoked by a finger movement. MEG has a high temporal resolution whereas fMRI allows the determination of active locations in 3-D space. Using beamformers and anatomical constraints we can estimate the activity as a function of time at these sites. The preprocessing steps necessary to meet these challenges (see Figure 2) include: Coregistration of the MEG, MRI and functional MRI data recorded from the same subjects. This is done via a transformation into a coordinate system defined by landmarks on the subjects head (nasion, left and right pre-auricular points marked with vitamin E capsules (a)); Extraction of the surface which defines the gray-white matter boundary (done in Freesurfer) and computing this boundary line on a given slice (b); Computing equally spaced normal vectors for the gray-white matter boundary (c). 2 k 1 g S k 1 k M 2 Θ and 2 k 1 (4) Unaveraged datasets usually contain a substantial amount of uncorrelated noise which can be characterized by a diagonal matrix Σ. Substituting C in (2) by C’=C+μΣ allows for controlling the sensitivity of the beamformer via the Backus-Gilbert parameter μ. Increasing μ leads to an increase in signal to noise ratio and to a decrease in the spatial resolution. Simply adding a constant to the diagonal elements of C creates an artificial noise floor, which represents a second possibility to ensure that the covariance matrix can be inverted, at the price of loosing spatial resolution. x (a) N GΘ Σ GΘ 2 Θ -1 1 g or N k 1 M 2 Θ 2 k (c) Because EEG also contains radial sources, it may serve as an independent check of these results (work in progress). Work supported by NINDS (grant NS39845), NIMH (grants MH42900 and MH01386) and the Human Frontier Science Program. according to (2) and (4), respectively. 2 Applying beamformers with anatomical constraints leads to better localization of source activity and higher resolution; The temporal activity in a group of sources indicates traveling waves which are expected from known cortical properties but need to be verified by other methods; 1 2 The normalized power from the source at Θ is then given by SΘ NΘ . The covariance matrix may be singular or ill-conditioned; Scanning a 5-dimensional space is computationally expensive; (b) fMRI The source power calculated from (2) or (4) cannot be used to compare activities obtained from different Θs because due to the constraint HΘ·GΘ=1 the gain of the beamformer depends on the location and orientation of the source. The power, therefore, needs to be normalized by a gain factor, which can be found by calculating the sensitivity of the sensor array to uncorrelated noise from that source. This noise power is given by y Fig. 2 Anatomical constraints Right: The spatial patterns for the beamformer and the forward solutions from the same locations. The orientation of a source is determined by anatomical constraints. Figure 4: Comparison between brain activity picked up by fMRI, and beamformers Left: fMRI pattern (top), same activity plotted on the normal vectors (middle), and as a function along the line of the gray-white matter boundary (bottom); Middle: Source power derived from SAM. The lines are plotted in the direction of maximum source power which in most cases is not perpendicular to the graywhite matter boundary (top). Source power along the boundary line (bottom); Right: Same with the use of the anatomical constraint that the orientation is always perpendicular to the gray-white matter boundary. Note that the power and locations are similar to the ones detected by SAM but less smooth going along the boundary. 1. J.C. Mosher, P.L. Lewis, R.M. Leahy: “Multiple Dipole Modeling and Localization from Spatio-Temporal MEG Data”, IEEE Trans. Biomed. Eng., 39: 541-557 (1992). 2. C.T. Tesche, M.A. Uusitalo, R.J. Ilmoniemi, M. Huotilainen, M. Kojola, O. Salomen: “Signal-space projection of MEG data characterize both distributed and well-localized neuronal sources”, Electroencephalography and Clinical Neurophysiology, 95: 189-200 (1995). 3. B.D. van Veen, W. van Drongelen, M. Yuchtman, A. Suzuki: “Localization of brain electrical activity via linearly constraint minimum variance spatial filtering”, IEEE Trans. Biomed. Eng., 44: 867-880 (1997). 4. S.E. Robinson, J. Vrba: “Functional neuroimaging by synthetic aperture magnetometry”, in: Recent Advances in Biomagnetism, T. Yoshimoto et al., eds., Tohoku University Press, Sendai, pp. 302-305 (1999).