Geometric Isomers
advertisement

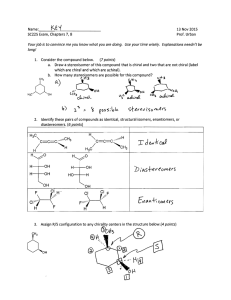
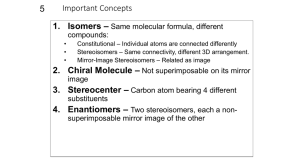
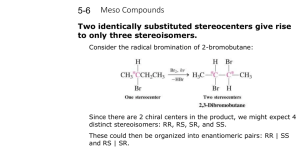
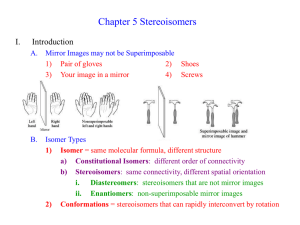
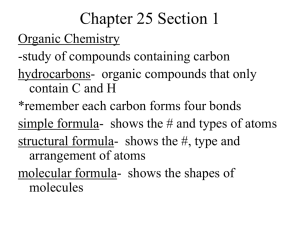
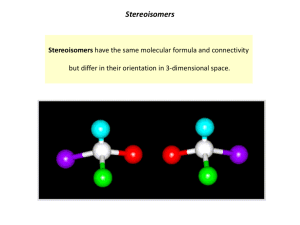
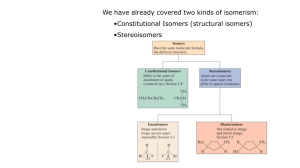
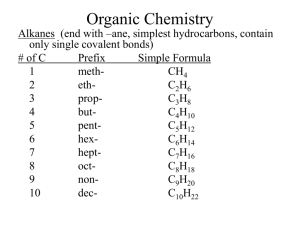
Geometric Isomers Lack of rotation around carbon-carbon double bonds has an important structural implication. These are not the same. Therefore, they cannot have the same name. cis-2-butene trans-2-butene If the hydrogens are on the same side it is a cis configuration. If they are on opposite sides of the double bond it is a trans configuration. Name each: Cis-2-pentene 1-pentene Trans-2-pentene Name it: C-C-C-C-C=C-C C H H 5-methyl-cis-2-heptene Stereoisomers Molecules of the same molecular structure that differ only in the arrangement of atoms in space. What does this mean? You need to understand 2 terms: 1. Superimposable Object is symmetrical - mirror image is no different then original. Ex- ball 2. Nonsuperimposable Mirror image cannot be placed on top of original Ex- your hands Stereoisomers A chiral carbon is asymmetrical when it has 4 different groups. ex - CHFClBr Where is the chiral carbon? C-C-C-C-C-C-C CC