PPT Notes 2 - Covalent Bonding & Lewis Structures
advertisement
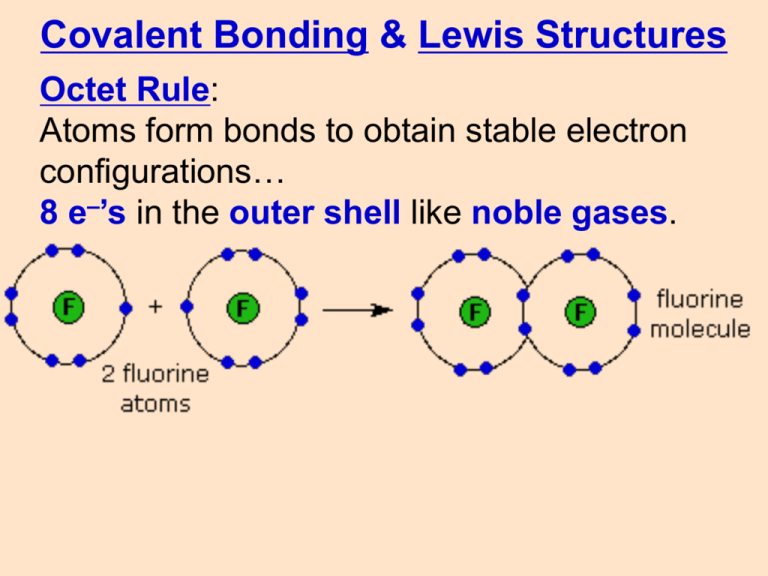
Covalent Bonding & Lewis Structures Octet Rule: Atoms form bonds to obtain stable electron configurations… 8 e–’s in the outer shell like noble gases. lone pair nonbonded pair unshared pair 1 shared pair 1 covalent bond single bond: 1 pair of shared e–’s structural formula: shows the arrangement of atoms How many unshared pairs? 2 How many shared pairs? 3 double bond: O O 2 pairs of shared e–’s triple bond: N N 3 pairs of shared e–’s Super Special Carbon C Carbon can form long chains because it forms up to 4 bonds on each C atom. This allows large biomolecules to form such as: proteins lipids (fats) carbohydrates (sugars/starches) nucleic acids (DNA/RNA) Drawing Lewis Structures In 5 easy steps: PCl3 1. 2. 3. 4. 5. Count Connect Octet Octet (if necessary) Drawing Lewis Structures PCl3 5 + 3(7) = 26 1. COUNT valence e–’s of all atoms in the molecule. Drawing Lewis Structures 2. CONNECT atoms to the central atom with single bonds. Central atom is the least electronegative (never H) Keep track of the electrons: 26 6 = 20 Drawing Lewis Structures 3. OCTET fill outer octets Keep track of the electrons: 26 6 = 20 20 18 = 2 Drawing Lewis Structures 4. OCTET fill central octet Keep track of the electrons: Draw the Lewis structure for HCN 26 6 = 20 22=0 20 18 = 2 Drawing Lewis Structures 5. If you run out of e–’s before the central atom has an octet… …form multiple bonds until it does. Drawing Lewis Structures In 5 easy steps: 1. 2. 3. 4. 5. Count (val e–’s) Connect (bonds) Octet (outer dots) Octet (central dots) (if necessary) form Multiple Bonds to fill central octet Draw the Lewis structure for trichloromethane, CHCl3 Draw the Lewis structure for carbon dioxide, CO2 O C O O C O Quick Quiz! 1. Carbon atoms have 4 valence electrons to form up to 4 bonds. This allows carbon to form ______________ needed for life. A. large, long chain biomolecules. B. nucleic acids C. carbohydrates D. ALL of the above Quick Quiz. 2. Which of the following diatomic molecules have a triple bond? (hint: draw the Lewis structures) A. O2 O O B. N2 N N C. Br2 Br Br D. H2 H H Quick Quiz. 3. Draw the correct Lewis structure for dinitrogen monoxide , N2O. (aka: nitrous or laughing gas) hint: arrange the atoms as… N N O N N O





