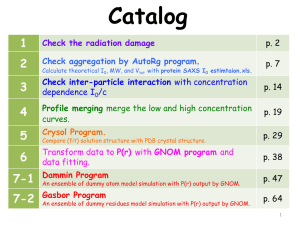
BioSAXS_check list (ppt)
advertisement

-1 -1 I(q) (cm ) 10 -2 10 -3 10 fitted solution envelope of native cyt. c -4 10 0.1 -1 q (Å ) Acknowledge EMBL Hamburg BioSAXS team memebers’ effort in ATSAS software package. The data analysis software, ATSAS software package, are downloaded from http://www.embl-hamburg.de/biosaxs/atsas-online/download.php If one use some programs in the work, please cite the corresponding papers. 1 G86_0 G86_1 G86_2 G86_3 G86_4 G86_5 G86_6 G86_7 G86_8 G86_9 combined 1 0.1 0.01 -1 I(q) (cm ) 1. Check the radiation damage 1E-3 1E-4 Cyt c-10 mg 15keV (10 sec / 10 frames) tm = 0.61124 calibrated thickness: 3.254 mm 1E-5 1E-6 0.01 0.1 1 -1 q (Å ) 2. Check aggregation by AutoRg program. Calculate theoretical I0, MW, and Vtot with protein SAXS I0 estimtaion.xls. 3. Check inter-particle interaction Cytc-10mg/ml Cytc-5mg/ml Cytc-2mg/ml 0.006 -1 -1 I(q)/c (cm mg ml) with concentration dependence I0/c 0.009 0.003 0.000 0.05 4. Profile merging merge the low and high concentration curves. 5. Crysol Program Compare (fit) solution structure with PDB crystal structure. 6. Transform data to P(r) with GNOM program and data fitting. 7. Dammin / Gasbor Program An ensemble of dummy atom/residues model simulation with P(r) output by GNOM. 0.10 -1 q (Å ) 0.15 0.20 1. Check the radiation damage Instruction: (1) Plot each frame and combined data. (2) Check the low-q region in the all profiles. • • If all profiles are overlapped well at low-q region, no radiation damage occurs. If some frames of them appear up-turn at low-q region, radiation damage may occur. The radiation damage might result from long data requisition time or aggregation sample. Resolving ❶ 1. combine the frames without up-turn and out a low-q reliable curve. 2. combine all frames and output high-q reliable curve. 3. fine-tune and merge the two curves and output into one profile. Resolving ❷ 1. Run HPLC G86_0 G86_1 G86_2 G86_3 G86_4 G86_5 G86_6 G86_7 G86_8 G86_9 combined 1 1 0.1 -1 I(q) (cm ) 0.01 1E-3 1E-4 1E-5 1E-6 0.01 0.1 1 -1 q (Å ) 2 G86_0 G86_1 G86_2 G86_3 G86_4 G86_5 G86_6 G86_7 G86_8 G86_9 combined 0.1 -1 I(q) (cm ) 0.08 0.06 0.04 0.02 0.02 well-overlapped 0.04 -1 q (Å ) 0.06 0.08 3 2. Check aggregation by AutoRg program. Instruction: (1) Start program: Primus -> AutoRg; input file type: txt, dat. (2) In Plot tab, see the raw data. (3) In Guinier tab, check the aggregation and define the qmin before unreliable range. (4) In Info tab, read the sRg limits (Guinier region), I0, and Rg values. 1 2 3 4 Define qmin before unreliable range. 4 One can calculate theoretical MW, I0, and Vtot with protein SAXS I0 estimtaion.xls. (spreadsheet edited by Dr. Jeng) Instruction: (1) Fill in protein amino acids numbers in AQ column. (2) Fill in protein concentration in B18 cell. (3) Fill in the aggregation number in B(13) cell. (4) Check the molecular weight calculated in B(17). (5) Check the calculated I(0) at B(19). (6) Input measured I(0) in B(27) to check the aggregation no. of oligomers. e.g. Cytochrome c (PDB ID: 1HRC), MW~12368 3 1 4 2 5 6 5 3. Check inter-particle interaction with concentration dependence I0/c. Instruction: (1) Plot all different concentration curves. (2) Confirm if concentration dependence is distinguishable at low-q region. e.g. There is concentration dependence behavior in below figure. The high conc. profile displays lower I0/c value due to the interference from inter-particle interaction. Cytc-10mg/ml Cytc-5mg/ml Cytc-2mg/ml 0.006 -1 -1 I(q)/c (cm mg ml) 0.009 0.003 inter-particle interaction 0.000 0.05 0.10 -1 q (Å ) 0.15 0.20 Attention! Need to measure low concentration sample as low as possible. Make sure that more than two I0/c curves are overlapped well. 6 4. Profile merging – merge the low and high concentration curves. Instruction: (1) Start program: Primus (2) Click “Tools” to select the low and high concentration data for merging. (3) Click “plot” to plot the two curves. 2 3 * * *nBeg value First 45 points in #1 and #2 are cut because of beamstop. 7 (4) According to the q-cut range (see ① and ②) , input the parameters into nBeg (begin point #) and nEnd (end point #) of Data Processing. (5) Click “Plot Scale ” and make sure that the curves are superimposable. ① remove concentration dependence 4 ② remove divergence region 0.009 0.006 -1 -1 I(q)/c (cm mg ml) cytc-5mg/ml cytc-10mg/ml 0.003 0.000 q1-cut range (high conc. Profile) 0.05 0.10 0.15 0.20 0.25 q2-cut range (low conc. profile) -1 q(Å ) Input q-cut region based on ① and ② The high-q endpoints of low conc. profile do not meet the other curve. 5 8 The scale multiplier is based on the intensity of the first file (#1) (6) Fine tune the eEnd point to make sure that the endpoints are superimposed with the merged curve. 6 Each Merge-click can output one merged file. Filename of 1st profile-merging: Merge00.dat Filename of 2nd profile-merging: Merge01.dat Attention! • Need to measure low concentration sample as low as possible. • Need to check concentration dependence before model simulation. 9 • Make sure that all different concentration curves are superimposable after q-cut. 5. Compare (fit) solution structure with PDB crystal structure by Crysol Program (in ATSAS package). Instruction: (1) Enter your option <0>: (2) Select the PDB file (protein data bank) (3) Maximum order of harmonics <15>: (4) Order of Fibonacci grid <17>: (5) Maximum s value <0.5>: (6) Number of points <51>: (7) Account for explicit hydrogens <no>: (8) Fit the experimental curve <yes>: (9) Enter data file (experimental dat file) (10) Subtract constant <no>: (11) Angular units in the input file <1>: (12) Electron density of the solvent <0.334>: (13) Plot the fit <yes>: (14) Another set of parameters <no>: (15) Press “ ” to terminate the program e.g. Cytochrome c (1HRC), MW~12368 2 9 Experimental SAXS data 10 (16) Plot the 1st (experimental scattering vector) and 2nd (theoretical intensity in solution) columns of fit file. (17) Open the log file to read the experimental and theoretical Rg values. Cyt c 2 Crysol fit, =2.576 -1 I(q) (cm ) 0.1 0.01 1E-3 fitted solution envelope of native cyt. c 1E-4 0.01 0.1 -1 q (Å ) Refer to http://www.embl-hamburg.de/biosaxs/manual_crysol.html#un 6. Transform data to probability distribution function P(r) with GNOM program and data fitting Instruction: (1) Printer type [ postscr ] : (2) Input data, first file : (select the file) (3) Output file [ gnom.out ] : filename.out (4) No of start points to skip [ 0 ] : (5) Input data, second file [ none ] : (6) No of end points to omit [ 0 ] : (7) Angular scale (1/2/3/4) [ 1 ] : (8) Plot input data (Y/N) [ Yes ] : (9) File containing expert parameters [ none ] : (10) Kernel already calculated (Y/N) [ No ] : (11) Type of system (0/1/2/3/4/5/6) [ 0 ] : (12) Zero condition at r=rmin (Y/N) [ Yes ] : (13) Zero condition at r=rmax (Y/N) [ Yes ] : (14) Rmax for evaluating p(r) : (input a proper value) (15) Number of points in real space [ 101 ] : (16) Kernel-storage file name [ kern.bin ] : (17) Experimental setup (0/1/2) [ 0 ] : (18) Initial ALPHA [ 0.0 ] : (19) Plot results (Y/N) [ Yes ] : (20) Press CR to continue probability distribution function P(r) In this case, Rmax (diameter of the object) = 40 Å 12 7. Dammin Program Dummy atom model simulation with P(r) output by GNOM program. (1) Mode: <[F]>ast, [S]low, [J]ag, [K]eep, [E]xpert < Fast >: (2) Log file name < .log >: filename .log (PLEASE SELECT THE INPUT FILE NAME) (3) Input data, GNOM output file name < .out >: filename File to be opened: m.out Blue-colored text Project identificator : m come from out file (4) Enter project description : read by Dammin Random sequence initialized from : 172235 ** Information read from the GNOM file ** Data set title: Merge of: cytc-5.txt cytc-10.txt Raw data file name: Merge01.dat Maximum diameter of the particle : 40.00 Solution at Alpha = 0.203E+01 Rg : 0.132E+02 I(0) : 0.388E-01 Radius of gyration read : 13.20 Number of GNOM data points : 428 χ2=1.179 (5) Angular units in the input file: 4*pi*sin(theta)/lambda [1/angstrom] (1) 4*pi*sin(theta)/lambda [1/nm ] (2) < 1 >: Maximum s value [1/angstrom] : 0.3924 Number of Shannon channels . : 4.996 Portion of the curve to be fitted < 1.000 >: Number of knots in the curve to fit : 20 *** Warning: constant reduced to avoid oversubtraction A constant was subtracted : 1.788e-4 Maximum order of harmonics : 10 (6) Initial DAM: type S for sphere [default], E for ellipsoid, C for cylinder, P for parallelepiped or start file name <.pdb >: (select one type) (7) Symmetry: P1...19 or Pn2 (n=1,..,12) or P23 or P432 or PICO < P1 >: (8) Expected particle shape: <P>rolate, <O>blate, or <U>nknown < unknown >: Start running simulation Dammin Output Files: 1. log file: contain the same information as the screen output. 2. -0.pdb file: contain the beads of the solvent inside the search volume 3. -1.pdb: represent the modeled particle. 4. fir file: Fit of the simulated scattering curve versus the experimental data. Columns in the output file are: 's', 'Iexp', 'Errexp'and 'Isim'. 13 7. Gasbor Program An ensemble of dummy residues model simulation with P(r) output by GNOM program. (1) Computation mode (User or expert)……<User>: (2) Log file name <.log>:filename .log ***Please Select The Input Filename*** File to be opened: m.out (3) Enter Project decsription: (4) Angular units in the input file: 4*pi*sin(theta)/lambda [1/angstrom] (1) 4*pi*sin(theta)/lambda [1/nm ] (2) < 1 >: (5) Portion of the curve to be fitted……<1.000>: (6) Initial DRM (CR for random)……< .pdb>: (7) Symmetry: P1…19 or Pn2 (n=1,..,12) or P23 or P432 or PIC0….< P1>: (8) number of residues in asymmetric part..< 62>: (9) Fibonacci grid order……<9>: (10) Expected particle shape: <P>rolate, <O>blate, or <U>nknown……<Unknown>: Cytochrome c (1HRC) Start running simulation Displayed by VMD χ2=2.286 View along x GASBOR Output Files: 1. log file: contain the same information as the screen output. 2. fir file: fit to the raw experimental data 3. pdb file: resulting model in PDB-like format that can be viewed with MASSHA (in ATSAS package) or VMD (molecular visualization program) View along y 14 View along z Appendix. On-line fit solution structure (experimental profile) with crystal structure (PDB or upload file). Instruction: (1) Open the FoXS website http://modbase.compbio.ucsf.edu/foxs/index.html (2) Input the PDB ID or upload the PDB file. (3) Upload the experimental profile. (4) Click “Submit Form”. FoXS: Fast SAXS Profile Computation with Debye Formula 15