Homework Answers
advertisement

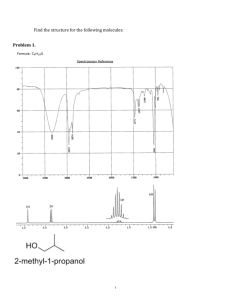
1) A compound gives a mass spectrum with peaks at m/z = 77 (40%), 112 (100%), 114 (33%), and essentially no other peaks. Identify the compound. First, your molecular ion peak is 112 and you have a M+2 peak at 114. Therefore, you have a halogen. Now, your molecular ion peak and M+2 peak are in a 3 to 1 ratio. This means chlorine. So, 112-35=77 # C’s 77/12=6 carbons so C6H5Cl. DOUS (2(6)+2-5-1)/2=4 Cl 2) While organizing the undergraduate stockroom, a new chemistry professor found a half-gallon jug containing a cloudy liquid (bp 100– 105 °C), marked only "STUDENT PREP". She ran a quick mass spectrum, which is shown below. As soon as she saw the spectrum (without even checking the actual mass numbers), she said, "I know what it is." What compound is the "student prep"? So molecular ion peak at 136 and M+2 peak at 138, so halogen present. They are in a 1:1 ratio so Br. So 136-79=57/12 = 4x12=48 57-48=9, C4H9Br (2(4)+2-9-1)/2=0 Br The peaks at 107 (C2H5) and 93(C3H7) tell us it is a linear chain instead of a branched one. 3) A laboratory student added 1-bromobutane to a flask containing dry ether and Mg turnings. An exothermic reaction resulted, and the ether boiled vigorously for several minutes. Then she added acetone to the reaction mixture, and the ether boiled even more vigorously. She added dilute acid to the mixture, and separated the layers. She evaporated the ether layer, and distilled a liquid that boiled at 143 °C. GC–MS analysis of the distillate showed one major product with a few minor impurities. The mass spectrum of the major product is shown below. Show the structure of this major product. Mg/ether MgBr Br O H+ OH O- The molecular ion peak should be at 116, but the loss of a carbon from the quatenary C forms a stable carbocation at 101. 1) One of the following compounds is responsible for the IR spectrum shown. Draw the structure of the responsible compound. 1-butene, 1-butanol, 4-hydroxy-1-butene, methyl propyl ether, butanoic acid. First thing to notice is the presence of an alcohol at 3200-3600 cm-1, which narrows our choices down to 1-butanol, 4-hydroxy-1-butene and butanoic acid.. O OH OH OH Only 1-butanol works, because there are no peaks corresponding to C=C and C=O. 2) One of the following compounds is responsible for the IR spectrum shown. Draw the structure of the responsible compound. phenylacetone, benzoic acid, acetophenone, benzyl alcohol, benzaldehyde First you can eliminate benzoic acid and benzyl alcohol because there is no –OH peak. Second you can eliminate benzaldehyde because there is no peak at 2740 cm-1 (aldehyde peak). That leaves phenylacetone and acetophenone. O O Acetophenone is the answer because the carbonyl peak is at 1700 cm-1 and a simple ketone like that on phenylacetone would absorb at a higher energy. 3) One of the following compounds is responsible for the IR spectrum shown. Draw the structure of the responsible compound. 2-ethynylcyclohexanone, 2-methyl-2-cyclohexenone, acetophenone, cyclohexylmethyl alcohol, 4-ethylcyclohexanone. First, 2-ethynylcyclohexanone can be eliminated because there is no peak for a carbon carbon triple bond. Second, cyclohexylmethyl alcohol is eliminated because there is no –OH peak present. Acetopheone can be eliminated next because there is no peak for aromatic C-H stretches. Also the carbonyl peak would be at a higher energy like around 1800 cm-1. 2-methyl-2-cyclohexenone can be eliminated because there is no O carbon carbon double bond peak. Leaving 4-ethylcyclohexanone. (4) Determine the structure of the compound that gives rise to the following mass and IR spectra. The molecular ion peak is at 162 and the M+2 peak is at 164, and they are in a 1:1 ratio, therefore there is a bromine atom. 162-79=83 83/12=6 carbons and 6x12= 72 so 83-72=11 C6H11Br So 2(6)+2-11-1=2/2=1, which means 1 double bond or 1 ring. Looking at the IR, there is no C=C peak so that means a ring. Br (5) Determine the structure of the compound that gives rise to the following mass and IR spectra. So the molecular ion peak is 72. So 72/12= 6 carbons 2(6)+2=14/2=7 a bit high. So subtract 1 C and replace with 12 Hs. C5H12. 2(5)+2-12=0 Not pentane, there is a carbonyl stretch. So to add an O, subtract a methyl group C4H8O. 2(4)+2-8=2/2=1 Lastly there is a peak at 2740 which tells us that the carbonyl is due to an aldehyde. O (1) The following 1H NMR spectrum is of a compound of molecular formula C3H8O. Propose a structure for this compound. First you have a septet that integrates to 1 H and a doublet that integrates to 6 Hs. This is typical of an isopropyl group. Then the peak at 2.5ppm. is a singlet and represents a H on an OH group. OH 2) Draw the structure of the compound with the 1H NMR and IR spectra shown and the formula C5H12O. 2(5)+2-12=0 so no double bonds or rings. Also there is no –OH or C=O peaks in the IR, so it has to be an ether. Looking downfield you have a triplet and a singlet. For there to be a singlet there must be only a methyl on one side of the ether. Thus giving us the following structure. O Looking at this structure, it explains the presence of the multiplets for the middle two CH2s. And finally the triplet upfield is for the terminal methyl group. 3) Draw the structure of the compound with the 1H NMR and IR spectra shown and the formula C6H12O2. First 2(6)+2-12=2/2=1 And based on the carbonyl peak in the IR we know this is our degree of unsaturation. Also we know that there must also be an ether since there is no OH peak in the IR. And based on the proton NMR we have two types of protons. One has to be connected to the ether. And for the rest to be all the same, there must be an isobutyl group. O O (4) Determine the structure of the following compound based on its mass, IR, and 1H NMR spectra. 114/12=9 carbons 114-108=6 hydrogens C9H6 2(9)+2-6= 7 degrees of unsaturation Based on the IR we know there is a carbonyl So –CH4 add O C8H2O 2(8)+2-2=16/2=8 Lets take off a C and add 12 Hs C7H14O 2(7)+2-14=2/2=1 Which accounts for the C=O 1) What is the major product of the following reaction? HBr Br H + Br Br 2) What is the major product of the following reaction? 1. Hg(OAc)2, H2O 2. NaBH4 , HO– H O + + HgOAc H HgOAc HgOAc HOH OH OH NaBH4 HgOAc 3) What is the major product of the following reaction? HBr peroxide Br Br H Br Br 4) What is the major product of the following reaction? Cl2 CH2Cl2 Cl Cl Cl Cl Cl Cl 5) What alkene, when allowed to react with HBr, would produce the following alkyl bromide? (There is more than one correct answer.) H Br Br Br 14.43) OCH3 a) H3C CH quartet OCH3 doublet O b) H2 C H3C O C H2 triplet triplet OCH3 e) doublet O H2 C H3C CH O CH3 triplet CH3 septet h) quartet multplet of 12 triplet H2 C H3C triplet H2 C C H2 multiplet of 9 OH singlet j) H3C H3C doublet H CH2 doublet H l) H3C H H H multiplet of 16 doublet of doublets doublet of doublets