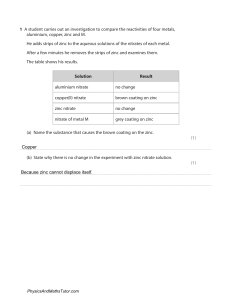
Formula
advertisement

Lab: Testing For Ions SNC 2D Name_____________ Period ____________ Date ____________ Observations Part 1 Solutions Iodide ( I-1) Color: Carbonate (CO3-2) Color: Phosphate (PO4-3) Color: Formula: Color: Formula: Color: Formula: Color: Formula: Color: Formula: Color: Formula: Color: Formula: Color: Formula: Color: Formula: Color: Formula: Formula: Formula: Silver (Ag+1) Barium (Ba+2) Lead (Pb+2) Iron (Fe+3) Observations Part 2 Tap Water/Testing Solutions Barium (Ba+2) Thiocyanate (SCN-1) Silver (Ag+1) Color: Color: Color: Formula: Color: Formula: Color: Formula: Color: Formula: Color: Formula: Color: Formula: Color: Formula: Color: Formula: Color: Formula: Color: Formula: Formula: Formula: -1 Chloride (Cl ) Sulfate ( SO4-2) Iron (Fe+3) Unknown # _____ MY UNKNOWN WATER SAMPLE #______ CONTAINS__________. 1a) Explain what is meant by a positive test for an ion. ( 1 mark ) b) Describe two types of changes that demonstrate a positive test. ( 2 marks ) 2. Write chemical formulas for the following substances. ( 2 marks ) a) silver nitrate ____________ b) sodium sulfate _____________ c) barium chloride_______________ d) iron (III) nitrate _______________ 3. Why do you think chemical tests, similar to the tests used in this investigation are called “qualitative “ tests? ( 1 mark ) 4. If silver nitrate solution is added to a potassium chloride solution and a precipitate forms, what are the names and formulas of the possible products? ( 2 marks ) 5. Suppose that you were asked to determine whether an ion was present in a solution and how much was present. ( 2 marks ) (Refer to Observation Table 2) a) Compare the amounts of precipitate that you would expect if you added barium chloride to two solutions that contained different amounts of sulfate ions. b) Compare the colour intensity that you would expect to see if you added potassium thiocyanate to two solutions that contained different amounts of iron (III) ions.