The Atom
advertisement
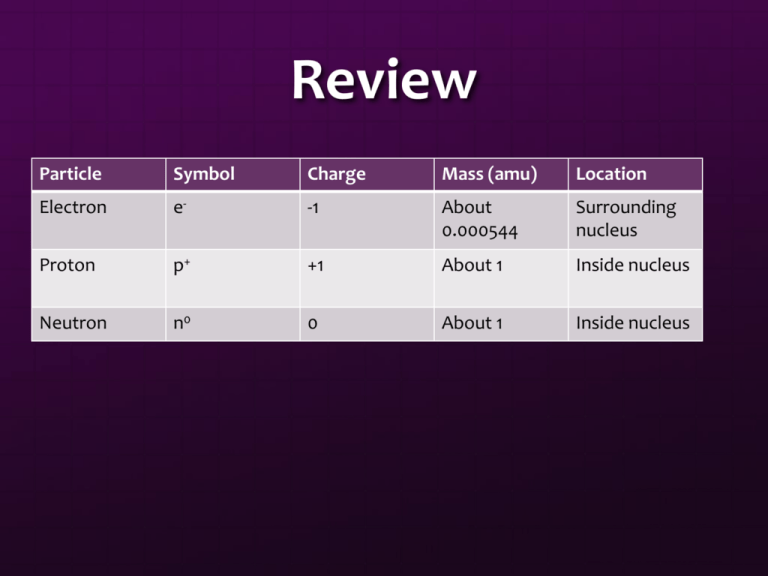
Review Particle Symbol Charge Mass (amu) Location Electron e- -1 About 0.000544 Surrounding nucleus Proton p+ +1 About 1 Inside nucleus Neutron n0 0 About 1 Inside nucleus Isotopes J.J. Thomson saw that there were two different neon atoms in the cathode ray tube These atoms were different in mass, but the same chemically Atoms of the same element that have a different mass are known as Isotopes Isotopes Contain the same number of protons Contain a different number of neutrons Two definitions for isotopes: Atoms of the same element with different amount of neutrons Atoms of the same element with difference in mass Isotope Uses Carbon dating Can determine the amount of carbon-14 in a sample to find the age of rock Smoke Detectors When smoke is present, conductivity in detector changes from small amount of radioactive material Cancer treatments Radiopharmaceuticals Henry Moseley Henry Moseley Found that the wavelength of X-rays depended on the number of protons in the nucleus of the atom Also found that the number of protons is always the same in an element Atomic number: number of protons in an element Protons: positive charge, mass: about 1 amu, symbol p+ Looking at the Numbers Nucleons: particles that make up the atomic nucleus Mass Number: the total number of nucleons (protons and neutrons) in an atom Neutrons: neutral charge, mass: about 1 amu, symbol n0 If you know this, you can figure out the number of protons, neutrons, and the mass number of anything! Writing Isotopes There are two main ways that isotopes can be written for an element. 126I Hydrogen-3 Isotopes of Neon Neon-20: 10 neutrons Neon-21: 11 neutrons Neon-22: 12 neutrons Atoms are Neutral The number of protons must equal the number of electrons for the atom to be neutral Isotopes change the number of neutrons Let’s Practice! An atom of vanadium contains 23 electrons. How many protons does it contain? An atom of silver contains 47 protons. What is its atomic number? How many electrons, neutrons, and protons are found in a copper atom of mass number 65? Atomic Mass On the periodic table, the AVERAGE atomic mass is given at the bottom This number represents the average mass of all of the isotopes for that particular element This number also involves the abundance of these particular isotopes in nature. Atomic Mass Calculations Calculate the average atomic mass of gold with 50% of gold being gold-197 and 50% being gold-198. Element Symbol Atomic Number Helium Ti # Electrons # Neutrons Mass Number 2 2 22 73 Nickel 108 28 Gallium 50 39 31 65 83 Bh 83 107 200





