ENGR 435
advertisement

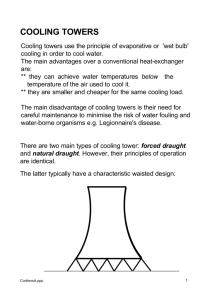
The University of Tennessee at Chattanooga College of Engineering and Computer Science Engineering 435 Chemical Process Systems Laboratory Portable Cooling Tower Team Members: Marc Moss Corita Suber ENGR 435-001 Dr. J. Cunningham September 22, 1998 Table of Contents II. Introduction III. Background and Theory IV. System Description V. Procedure A. Fan SSOC B. Heat Transfer (Steady State) VI. Results VII. Discussion VIII. Conclusion and Recommendations IX. References X. Appendix A. Raw Data B. Sample Calculations Introduction The members of this group spent three weeks analyzing the Portable Cooling Tower located in Room 115 of Grote Hall. The airflow rate was analyzed to construct a steady operating curve. In addition, the energy flow rate was examined from both the air and water sides. This report discusses the procedure followed while performing the experiments. It begins with a description of the Portable Cooling Tower. Along with the theory of how it works. It is followed by the experimental procedures and the results obtained. The report is concluded with a discussion of the significance of the experiment and the results, as well as the conclusion drawn. An appendix is attached to the end of the report and includes the raw data charts and references. Background and Theory The governing principles behind the operation of the cooling tower is the First Law of Thermodynamics, which states that energy can neither be created nor destroyed, only converted from one form into another. The cooling tower does this by transferring thermal energy stored in hot water to a counter current of ambient air supplied by means of forced convection. The inlet and outlet temperatures of both the air and water can be recorded and used to determine the enthalpy changes of the two streams. These enthalpy changes of the two streams, along with the flow rates, can then be used to calculate the heat transfer (q) for each stream from the following equation: q M H This is an idealized calculation of the heat transfer, and does not account for losses in energy due to friction and conduction through the walls of the cooling tower. These values cannot be easily quantified individually, but the total value of the losses can be determined by finding the difference between the q value for air and the one of water. However, losses are considered to be minimal and are ignored, even though this is a poor assumption. System Description The portable cooling tower (See Figure 1) operates by pumping a supply of cooling water out of a reservoir into the customers load unit. This cooling water supply (CWS) is then used to cool this load unit. The warmed cooling water (CWR) is then sent back to the cooling tower. The warm water enters the top of the tower and is sent through a rotating dispersion mechanism that sprays the water out over a series of baffles that fill the cooling tower. The water runs down these baffles and returns to the reservoir at the base of the tower. A fan attached to the side of the tower blows air up through the baffles, where it interacts with and cools the water flowing the other way. The system is controlled by means of the “LabView” computer program installed on a personal computer. “LabView” can be used to independently control the pump and fan motor speeds to run the systems as desired. “LabView” records the temperatures of the air and water streams, as well as the flow rate of the water. The humidity readings for the inlet and outlet air were determined by manually using an electronic hygrometer. The air velocities used to determine the air flow rate which were also taken by hand. Procedure 1: Fan SSOC As the air flow rate is not measured by the computer, it was decided to construct a steady state operating curve of the air flow rate versus percent fan motor speed. This was done by dividing the surface area of the top of the cooling tower where the air exits into three wedges of equal area. It was decided on three since this is how the cooling tower is partitioned by the construction. Each of these three wedges was divided into five, two inch bands (See Figure 2). The air velocity was measured on the inside and outside edges of these bands. This average was then multiplied by the area of the band to give a total flow rate. This procedure was done four times; at 40%, 60%, 80%, and 100% of the fan motor speed. It was done for the lower percentages because the velocities only became measurable in the range from 30%-40%. These four data points were then plotted to yield the SSOC. Results 1 Table 1 contains the measured air velocities that were used to plot the fan motor SSOC, which appears on the next page (Graph 1). Procedure 2: Heat Transfer (Steady State) A steady state analysis of the heat transfer was done on the system, with the fan motor operations as 80% and the hot water being supplied from the hot water line in Grote Hall. Since the hot water was a continuous supply from a separate source, the water was allowed to drain out of the reservoir after it had gone through the cooling tower. The water was turned on and the system was started and allowed to run at steady state. The computer read the stream temperatures and the inlet and outlet humidities were read by hand. The system ran for twelve minutes before the computer malfunctioned and stopped recording data. Only on run was made because of continuing difficulties with the computer. Results 2 Table 2 contains the average data for the heat transfer analysis. Plots of the air and water data can be found on the following pages (Graph 2 &3). The two graphs plot all of the data for the twelve minute run, but only the steady state range (from t=2min to t=8min) was used in the calculations Air In Air Out Water In Water Out Temp (C) Temp (F) Humidity Enthalpy 27.1 80.9 50% 31.9 38. 7 101.6 91% 62.2 51.1 123.9 52.2 29.0 84.2 91.9 Water Heat Transfer 3207 Btu/min Table 2: Heat Transfer Data Discussion The SSOC derived from the velocity data could be very useful in further runs using the system. Having an operating curve would allow the users to determine the air flow rate without having to measure the air velocity every time the system is used. Even though data was only recorded for one run, the plots of the air and water temperatures are useful for demonstrating the general behavior of the system. By sheer happenstance, the air inlet temperature experienced a sharp increase at a point approximately seven minutes into the experiment. A comparison of the plots of the air inlet temperatures and the CWS temperature at this time demonstrate the effect varying ambient temperatures have on the cooling tower’s ability to cool the water. At the point where the air temperatures increases, the CWS temperature also increases, revealing that the tower is not able to cool effectively at higher air temperatures. Conclusions and Recommendations The differences in the heat transfer rates for the air and water flows show that the various heat losses in the system are significant and should not be ignored. One recommendation for future experiments is to analyze these losses with varying system conditions (i.e. flow rates and water temperatures). References Felder, Richard M. and Ronald W. Rousseau. Elementary Principles of Chemical Processes, 2nd ed. John Wiley and Sons, New York, 1986. McCabe, Smith and Harroit. Unit Operations of Chemical Engineering, 5th ed. McGraw-Hill, New York, 1993. Appendix