Study Investigators and Supplemental Table and
advertisement

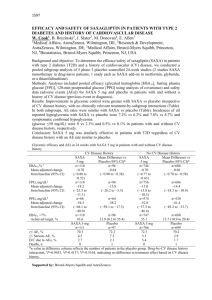
ONLINE APPENDIX Saxagliptin 014 Study Group STUDY INVESTIGATOR LIST Argentina: Bruzone S, Jadzinsky M, Libman A, Moreno M, Sinay I, Solis S, Sposetti G, Ulla MR; Australia: Karrasch J, Lowy A, Moses R, Phillips P, Roberts A, Stuckey B; Brazil: Forti A, Gross JL, Hissa M, Saraiva JF, Sgarbi JA, Soares FJ, Zanella MT; Canada: Aronson R, Bose S, Carlson B, Gervais B, Godsell S, Goldenberg R, Hart R, Herman D, Hramiak I, Lau D, Loader K, Lowe D, Mackinnon R, Mazza G, Misik K, O'Keefe D , O'Mahony M, Saliba N, Sinclair D, Twum-Barima DY, Wade A, Willoughby P, Woo V; Chile: Humphreys J, Maiz A; Mexico: Aguilera M, Alvarado R, De La Garza NE, Diaz A, Laviada H, Robles FJ, Rodriguez H, Sanchez HA, Zuñiga S; Puerto Rico: Abreu-Feshold F, Claudio J, Sosa-Padilla M, Vazquez-Tanus J, Velazquez-Navarro S; Taiwan: Chang CT, Li JC, Pei D, Sheu WH; United States: Agnone Jr. FA, Ahmann A, Awan N, Barrera J, Behnke A, Bonabi G, Brinson AC, Carson R, Carter K, Chappel C, Chrysant S, Cohen K, Cohen L, Corbett B, Davis J, DeFronzo R, Diederich C, Dimonte R, Dobs A, Doolan R, Downey HJ, Dyck D, Fears J, Fields H, Fox K, Free A, Furman MJ, Ganong KD, Garber A, Goldberg R, Graff A, Gray W, Green S, Hallmark BD, Harrison B, Hermansen E, Herring C, Horstmeyer P, Hurley D, Isakov T, Jacks W, Jacob P, Jacqmein J, Kayne D, Kayota S, Kim E, Klein E, Klocek J, Landgarten S, Latham G, Levins J, Lipetz R, Littlejohn TW, Lucas KJ, Lutz K, McManus S, McNeill R, Miller S, Miser W, Mitchell JR, Montoro R, Morales L, Nardandrea J, Norwood P, Oates S, Pajnigar A, Phillips F, Poindexter A, Pool JL, Rendell M, Rhudy J, Rigonan K, Robbins J, Roberts K, Robinson JG, Rosenstock J, Ruiz EA, Schwartz S, Selam JL, Shockey G, Shubrook J, Shue R, Simon H, Sloan GK, Smith TR, Spangenthal S, Spence J, Stanley R, Stanton D, Sugimoto DH, Suwannasri R, Tarshis G, Turner MJ, Udani J, Von Seggern R, Wayne J, Whiles R, Williams HT, Wingert K, Witkin DB, Woodward WR, Zayed A. 1 Table A1—Demographic and baseline characteristics of patients with type 2 diabetes by randomized group SAXA + MET 5 mg + MET n = 191 PBO + MET n = 179 2.5 mg + MET n = 192 54.8 (10.2) 26 (14.5) 54.7 (10.1) 33 (17.2) 54.7 (9.6) 32 (16.8) 54.2 (10.1) 26 (14.4) Sex† Male Female 96 (53.6) 83 (46.4) 83 (43.2) 109 (56.8) 103 (53.9) 88 (46.1) 95 (52.5) 86 (47.5) Race† Caucasian African American Asian Other 150 (83.8) 7 (3.9) 4 (2.2) 18 (10.1) 153 (79.7) 8 (4.2) 8 (4.2) 23 (12.0) 159 (83.2) 11 (5.8) 3 (1.6) 18 (9.4) 144 (79.6) 14 (7.7) 5 (2.8) 18 (9.9) Body weight (kg)* 87.1 (17.8) 86.0 (17.6) 87.3 (17.0) 87.8 (18.9) BMI (kg/m2)* 31.6 (4.8) 31.7 (5.2) 31.2 (4.7) 31.1 (4.8) Diabetes duration (years)* 6.7 (5.6) 6.7 (5.6) 6.4 (4.7) 6.3 (4.4) A1C (%)* 8.1 (0.9) 8.1 (1.0) 8.1 (0.8) 8.0 (1.0) FPG (mg/dl)* 174 (44) 174 (44) 180 (47) 176 (50) Characteristics Age (years)* ≥65 years† 10 mg + MET n = 181 † *Values are expressed as mean (SD). Values are expressed as n (%). SAXA: saxagliptin; MET: metformin; PBO: placebo; BMI: body mass index; FPG: fasting plasma glucose. 2 Figure A1—Patient disposition*† SAXA: saxagliptin; MET: metformin. *Patients who discontinued prematurely were required to have all final visit procedures performed at the time of discontinuation. † Progressively strict glycemic control criteria were defined for rescue therapy if fasting plasma glucose (FPG) was >240, >220, and >200 mg/dl for weeks 4–6, week 8, and weeks 12–24, respectively. Patients meeting these rescue criteria were entered directly into the extension period, in which they were administered open-label pioglitazone 15 mg (which could be titrated upward to 45 mg) in addition to blinded study medication plus open-label metformin. The number of patients who discontinued and who were rescued are not mutually exclusive. ‡ One patient was randomized directly after enrollment. This patient did not enter the lead-in period. 3 Figure A2 (composite figure) Figure a—Composite adjusted mean change from baseline to week 24 in postprandial insulin and C-peptide AUC a. Adjusted mean change from baseline in postprandial insulin AUC (LOCF) at 24 weeks b. Adjusted mean change from baseline in postprandial C-peptide AUC (LOCF) at 24 weeks LOCF: last-observation-carried-forward. 4