Unit 2: Energy
advertisement
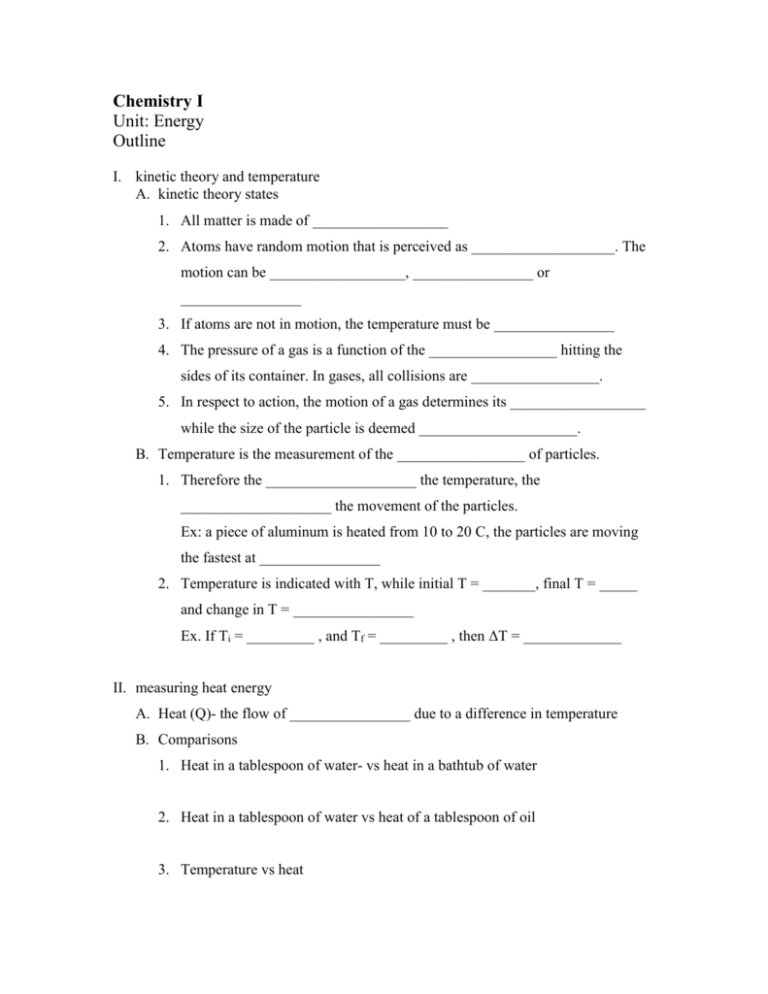
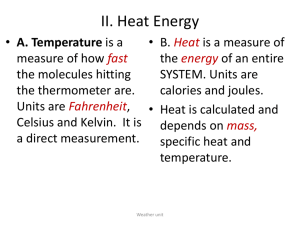
Chemistry I Unit: Energy Outline I. kinetic theory and temperature A. kinetic theory states 1. All matter is made of __________________ 2. Atoms have random motion that is perceived as ___________________. The motion can be __________________, ________________ or ________________ 3. If atoms are not in motion, the temperature must be ________________ 4. The pressure of a gas is a function of the _________________ hitting the sides of its container. In gases, all collisions are _________________. 5. In respect to action, the motion of a gas determines its __________________ while the size of the particle is deemed _____________________. B. Temperature is the measurement of the _________________ of particles. 1. Therefore the ____________________ the temperature, the ____________________ the movement of the particles. Ex: a piece of aluminum is heated from 10 to 20 C, the particles are moving the fastest at ________________ 2. Temperature is indicated with T, while initial T = _______, final T = _____ and change in T = ________________ Ex. If Ti = _________ , and Tf = _________ , then ΔT = _____________ II. measuring heat energy A. Heat (Q)- the flow of ________________ due to a difference in temperature B. Comparisons 1. Heat in a tablespoon of water- vs heat in a bathtub of water 2. Heat in a tablespoon of water vs heat of a tablespoon of oil 3. Temperature vs heat C. Units- measurement of the amount of ________ required or available for transfer a. Joules, kilojoules- 4.184 joules is equal to the amount of __________ required to heat one gram of water one degree Celsius b. calories, Calories (kilocalories) – one calorie is equal to the amount of _____________required to heat one gram of water one degree Celsius c. BTU = ___________________________________ d. Conversions Ex: Convert 350.1 J to calories Convert 5030 kcal to J Convert 900.0 kJ to cal D. Specific heat capacity (s) or (Cp) – quantity of energy required to raise _______ gram of any substance _________ degree Celsius. a. Valuesi specific heat of water is 4.184 J/gC OR 1.000 cal/gC ii specific heat of ___________ is _____________ iii specific heat of ___________ is ______________ b. Calculating heat i Equation Q = (m)(∆T)(s) m= ∆T = s= ii Applications Ex: How much energy is required to heat 20.0 grams of water from 15.6 C to 26.3 C? Ex: what is the specific heat of a metal if it takes 26.3 joules to heat 1.4 grams 15.2 C? what is this metal? Ex: what is the final temperature of a 5.3 gram sample of iron that loses 500.2 J of energy, if the iron beginning temperature is 120.0 C? III. energy transfer 1. Law of Conservation of energy (Q1 = Q2), states that energy cannot be _____________________ nor __________________________, but can be ______________________________. 2. Using LCOE to calculate variables (m1)(∆T1)(s1) = (m2)(∆T2)(s2) Ex: If 100.0 grams of water is heated 45 C, what amount of zinc must have lost 32 C? IV. The ‘energy curve’ Endothermic 1. Graph shape a. Temperature change- temperature is ________________________, energy is being used to ___________________________________________ b. Phase change- temperature is ___________________, energy is being used to overcome ____________________________ ________________ 2. Reading temperatures a. Normal boiling/condensation point of water is _________________ b. Normal freezing/ ____________ point of water is _______________ 3. Reading energy a. Heat of fusion- the amount of energy required to ___________ one gram (or one mol) of any substance Ex: the heat of fusion of ______________ is ________________ b. Heat of vaporization- the amount of energy required to _______________ one gram (or one mol) of any substance