Mass to Mass Stoichiometry
advertisement
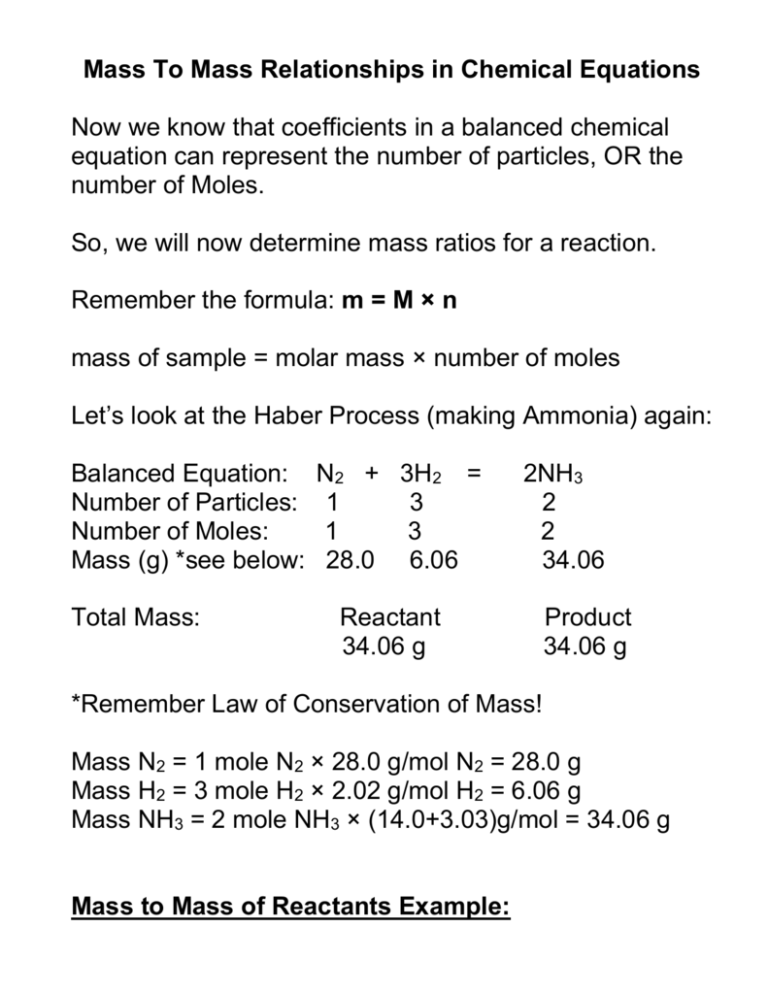
Mass To Mass Relationships in Chemical Equations Now we know that coefficients in a balanced chemical equation can represent the number of particles, OR the number of Moles. So, we will now determine mass ratios for a reaction. Remember the formula: m = M × n mass of sample = molar mass × number of moles Let’s look at the Haber Process (making Ammonia) again: Balanced Equation: Number of Particles: Number of Moles: Mass (g) *see below: Total Mass: N2 + 3H2 = 1 3 1 3 28.0 6.06 2NH3 2 2 34.06 Reactant 34.06 g Product 34.06 g *Remember Law of Conservation of Mass! Mass N2 = 1 mole N2 × 28.0 g/mol N2 = 28.0 g Mass H2 = 3 mole H2 × 2.02 g/mol H2 = 6.06 g Mass NH3 = 2 mole NH3 × (14.0+3.03)g/mol = 34.06 g Mass to Mass of Reactants Example: In outer space, CO2 is produced by astronauts and must be removed with LiOH to produce Lithium carbonate and water. An astronaut, on average, produces 1.00 × 103g CO2 per day. What mass of Lithium hydroxide is needed per astronaut each day? Answer: Step 1: Write a Chemical reaction CO2 + LiOH = Li2CO3 + H2O Step 2: Balance your chemical reaction CO2 + 2LiOH = Li2CO3 + H2O Step 3: Given the number of grams of CO2, calculate the number of moles of CO2 (we want to work with Mole ratios!) m=M×n 1.00 × 103 g = (12.00 g/mol + (2 × 16 g/mol)) × n 1.00 × 103 g = 44.00 g/mol × n n = 22.7 mol CO2 Step 4: Use mole ratios to find # of moles of LiOH Known ratio = Unknown Ratio 2 mol LiOH = x mol LiOH 1 mol CO2 22.7 mol CO2 Cross Multiply and solve for x (22.7 mol CO2) × 2 mol LiOH = x mol LiOH 1 mol CO2 45.4 mol LiOH = x mol LiOH Step 5: convert number of moles of LiOH to grams m=M×n m = 45.4 mol LiOH × (6.90 + 16.00 + 1.01)* Look up #s m = 45.4 mol LiOH × 23.91 g/mol LiOH m = 1085.06 g Therefore, 1.09 × 103 g of LiOH will react with 1.00 × 103 g CO2 ************************************************************* Summary: 1) Balanced Chem reaction 2) grams to moles (m = M × n) 3) mole : mole ratio (to find unknown) 4) moles to grams (m = M × n) **************************************************************** Homework: Page 244 Questions: 11, 12 and 13









