properties of aniline
advertisement

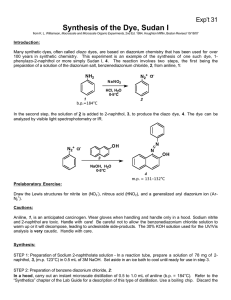
Experiment 4 Reactions of phenylamine (aniline) Write down the observations of the following experiments and give explanation with chemical equations, if appropriate. A. Reaction of the amino (NH2 group) 1. Indicator: To 2 drops of aniline in a test tube, add 5 drops of water. Insert a piece of pH paper ( or read litmus paper ) and shake. Note any colour change on the paper. Write down your observation. 2. Reaction with hydrochloric acid: (a) Place 5 drops of aniline in a test tube, and add 5 drops of water. Shake the mixture. Write down your observation. (b) Add concentrated hydrochloric acid dropwise until in excess. (c) Boil off the water and observe the residue. 3. Reaction with nitrous acid: In room temperature, place 3 drops of aniline in a test-tube, and add concentrated hydrochloric acid dropwise until a clear solution is formed. Dilute to 5 cm3 with water. Add 1 or 2 crystal of sodium nitrite, and warm gently. 4. Reaction with glacial ethanoic acid: Mix 1 of aniline and 1 of glacial ethanoic acid, and heat with a small bunsen flame. (* glacial ethanoic acid has a pungent choking smell, this experiment must be carried out in fume-cupboard. ) B. Reactions of the phenyl (C6H5 group) Place 5 drops of aniline in a test tube, and add concentrated hydrochloric acid dropwise until the aniline dissolves. Add bromine water drop by drop until in excess. C. Preparation and reactions of diazonium salts 1. Preparation of benzene diazonium chloride: Place 3 cm3 of aniline in a boiling tube. Add 10 cm3 of water, then 8 cm3 of concentrated hydrochloric acid. Cork the tube, and shake to dissolve the aniline hydrochloride. Remove the cork and insert the tube in a beaker containing a freezing mixture of ice, salt and water. The solution must be cooled below 5C. Make up a solution of 3 g of sodium nitrite in 8 cm3 of water, and cool this solution to 5C before adding it to the aniline solution, take great care that the temperature of solution does not rise above 10C. The diazonium solution is used for the reactions without further purification. 2. Reactions of benzene diazonium chloride: (a) Replacement reactions (Replacement of N+N Cl by OH group): Transfer 2 cm3 of the diazonium chloride solution to a test tube, and boil it gently. Observe the liquid forked and note it smell. (b) Coupling reactions (Formation of azo dyes): 1. Transfer 2 cm3 of the diazonium chloride solution to a test tube. Add aniline, previously cooled to below 5C, dropwise. Observe the formation of precipitate. 2. Dissolve 2 crystals of phenol in 2 cm3 of dilute sodium hydroxide solution. Cool this solution to below 5C and add the diazonium solution dropwise. Observe the formation of precipitate. 3. Repeat experiment (2), using 2-naphthol instead of phenol.