Midterm Exam Answer Key
advertisement
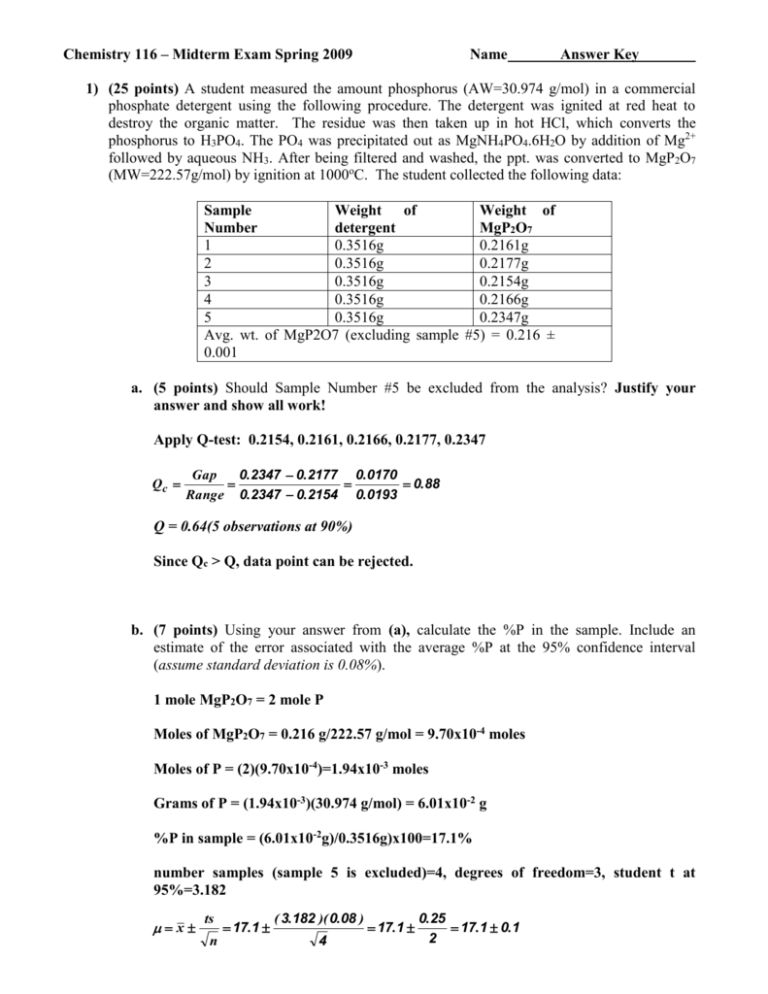
Chemistry 116 – Midterm Exam Spring 2009 Name Answer Key 1) (25 points) A student measured the amount phosphorus (AW=30.974 g/mol) in a commercial phosphate detergent using the following procedure. The detergent was ignited at red heat to destroy the organic matter. The residue was then taken up in hot HCl, which converts the phosphorus to H3PO4. The PO4 was precipitated out as MgNH4PO4.6H2O by addition of Mg2+ followed by aqueous NH3. After being filtered and washed, the ppt. was converted to MgP2O7 (MW=222.57g/mol) by ignition at 1000oC. The student collected the following data: Sample Weight of Weight of Number detergent MgP2O7 1 0.3516g 0.2161g 2 0.3516g 0.2177g 3 0.3516g 0.2154g 4 0.3516g 0.2166g 5 0.3516g 0.2347g Avg. wt. of MgP2O7 (excluding sample #5) = 0.216 ± 0.001 a. (5 points) Should Sample Number #5 be excluded from the analysis? Justify your answer and show all work! Apply Q-test: 0.2154, 0.2161, 0.2166, 0.2177, 0.2347 Qc Gap 0.2347 0.2177 0.0170 0.88 Range 0.2347 0.2154 0.0193 Q = 0.64(5 observations at 90%) Since Qc > Q, data point can be rejected. b. (7 points) Using your answer from (a), calculate the %P in the sample. Include an estimate of the error associated with the average %P at the 95% confidence interval (assume standard deviation is 0.08%). 1 mole MgP2O7 = 2 mole P Moles of MgP2O7 = 0.216 g/222.57 g/mol = 9.70x10-4 moles Moles of P = (2)(9.70x10-4)=1.94x10-3 moles Grams of P = (1.94x10-3)(30.974 g/mol) = 6.01x10-2 g %P in sample = (6.01x10-2g)/0.3516g)x100=17.1% number samples (sample 5 is excluded)=4, degrees of freedom=3, student t at 95%=3.182 x ts n 17.1 ( 3.182 )( 0.08 ) 4 17.1 0.25 17.1 0.1 2 Chemistry 116 – Midterm Exam Spring 2009 Name Answer Key c. (6 points) After completing the analysis, the student realized that she had weighed out approximately 0.37g of detergent for sample #5. i. (3 points) What type of error is this? systematic ii. (3 points) If sample #5 is included in the analysis, how would this affect the precision and accuracy of the results? The precision and accuracy would both decrease. d. (7 points) A second student performed the identical set of experiments (no data points were excluded) and obtained an average %P of 2.6%. Are the results of the two students significantly different at the 95% confidence interval (assume a pooled s of 0.3%)? Justify your answer and show all work! Apply Student’s t-test: Degrees of freedom = 4 + 5 -2 = 7 (sample #5 was discarded by the first student) t = 2.365 (95% confidence interval with 7 degrees of freedom) x x2 t calculated 1 s pooled n1 n2 17.1 2.6 n1 n2 0 .3 ( 4 )( 5 ) 20 48.3 48.3 2.22 72 (4 5 ) 9 Since tc > t, the students’ results are significantly different (different detergent sample?) Chemistry 116 – Midterm Exam Spring 2009 Name Answer Key 2) (25 points) Given the following calibration curve for measuring atmospheric CO2: a. (10 points) Calculate the concentration of CO2 in a sample with an average signal of 173 ± 7. Assume the error in the slope is ± 0.0008 and y-intercept is ± 0.0005. (Please make sure to propagate the error in your answer.) [CO2] = (S + 2.3306)/0.9292 = (173 + 2.3306)/0.9292 = 175.3306/0.9292 = 189 ppm Error: First addition: Second division, convert to relative uncertainty Relative uncertainty: 189 ppm ± 4% Absolute Uncertainty: (0.04)(188) = 8 189 ± 8 ppm Chemistry 116 – Midterm Exam Spring 2009 Name Answer Key b. (5 points) A second sample yielded an average signal of 415 ± 8, should the given calibration curve be used to determine the concentration of CO2 in this sample? Explain. No, the signal is significantly outside the calibration range and it is uncertain if the response will continue to be linear at higher CO2 concentrations. c. (5 points) If the standard deviation of the data points used to measure the calibration curve is 9.2, what is the minimum detectable concentration or detection limit? c 3 s ( 3 )( 9.2 ) 27.6 29.7 30 ppm m 0.9292 0.9292 m is the slope from the calibration curve d. (5 points) The CO2 calibration curve was determined using an internal standard. The area of the CO2 peak in an infrared spectrum was compared to the area of chloroform. If the response factor is 0.1563 and 100 ppm of chloroform yielded a peak area of 499.9, what concentration of CO2 would yield a peak are of 151.6? Ax A 1 A 151 .6 1 F S X S x ( 100 ppm ) ( 100 ppm )0.3033 6.398 X S AS F 499 .9 0.1563 X 194 Chemistry 116 – Midterm Exam Spring 2009 Name Answer Key 3) (20 points) Given the following complex equilibrium related to the solubility of BaC2O4(s): a. (4 points) Write out the charge balance equation. 2[Ba2+] + 1[H+] = 2[C2O42-] + 1[HC2O4-] + [OH-] H2C2O4 is uncharged. b. (6 points) Write out the mass balance equation. [Ba2+] = [C2O42-] Need to account for all sources of C2O42-: [Ba2+] = [C2O42-] + [HC2O4-] + [H2C2O4] c. (3 points) How many unknowns and equations are associated with determining the solubility of BaC2O4(s) (you don’t need to write out the equations and unknowns, just give a count)? Is this problem solvable? There are 6 unknowns: [Ba2+], [H+], [C2O42-], [HC2O4-], [H2C2O4], [OH-] and six equations: charge balance, mass balance and four equilibrium constants. Since the number of equations and unknowns are equal the problem is solvable. Chemistry 116 – Midterm Exam Spring 2009 Name Answer Key d. (7 points) What assumptions can be made to simplify the calculation? Assume activity coefficients are 1. Since Kb1 and Kb2 are so small, can assume [HC2O4-], [H2C2O4] are negligible. Ignore these two equations. Similarly, can assume [H+] = [OH-] = 1 x10-7 since [OH-] from bases are negligible. Reduces to two unknowns [Ba2+] , [C2O42-] and two equations (CB and MB are now the same). Ksp and CB/MB [Ba2+] = [C2O42-] 4) (20 points) a. (8 points) Calculate the pH for a solution containing 0.03M of benzoic acid (pKa = 4.202) and 0.01M of KCl (Please use activity coefficients). Ka = e-pKa = e-4.202 = 6.281x10-5 Ionic strength = 0.01M, , pH = - log [H+] = -log[( 0.914)(1.506x10-3)]= -log(1.376x10-3) = 2.86 Chemistry 116 – Midterm Exam Spring 2009 Name Answer Key b. (3 points) The addition of KCl increases the pH of the sample, is this an example of the common ion effect? No, the common ion effect occurs if the salt has an ion in common with the equilibrium. Benzoic acid does not contain either a K+ or Cl-. c. (3 points) If a base is added to the above solution of benzoic acid and KCl, what would happen to the relative concentrations of benzoic acid and benzoate? The concentration of benzoic acid would decrease and benzoate would increase. The base removes H+ and from Le Chatelier’s principal the equilibrium would shift to the right. d. (6 points) If a titration curve is obtained by measuring the change in pH as a function of the volume of a base that is incrementally added to the benzoic acid solution, what will determine the pH before, at and after the equivalence point? (use the Ag+ titration curve that was discussed at length in class and was based on the solubility of AgI(s) as a reference point or analogy to answer this question). Before equivalence: [benzoic acid] and Ka At equivalence: Ka After equivalence: [base] Chemistry 116 – Midterm Exam Spring 2009 Name Answer Key 5) (10 points) A 0.010M solution of EDTA is used to titrate 500 mL of a 0.0003M Ba2+ (Kf = 7.59x107) solution at pH 5. a. (7 points) What is pBa2+ at the equivalence point? EDTA and Ba+2 form a 1:1 molar complex At equivalence point number of moles of EDTA = moles of Ba2+ and all Ba2+ is in the form BaY2(x mL EDTA)(0.01M EDTA) = (500 mL)(0.0003M) x mL = (500 mL)(0.0003M)/(0.01M EDTA) = 15 mL Account for dilution: [BaY2-] = (0.0003M)(500 mL)/(515 mL) = 2.913x10-4 Concentration of Ba+2 depends on Kf K 'f K f Y 4 [ Ba( EDTA ) -2 ] [ Ba 2 ][ EDTA] ( 7.59 10 7 )( 2.9 x10 7 ) ( 2.913 x10 4 x ) 22.01 x 2 x 2.913 x10 4 0 ( x )( x ) solve quadratic equation : x [ Ba 2 ] 2.89 x10 4 pBa 2 log( 2.89 x10 4 ) 3.54 b. (3 points) Xylenol orange (In) is used as an indicator: BaIn + EDTA BaEDTA + H3In3(red) (yellow) What is the color of the solution at the end-point? Color changes from red to yellow. Indicator binds Ba+2 weaker than EDTA. As long as there is excess Ba+2 the indicator will form a complex with Ba+2, but at the equivalence point all Ba+2 will be in complex with EDTA and the indicator will be free and undergo a color change.







