revised lesson plan over Avogadro's law
advertisement

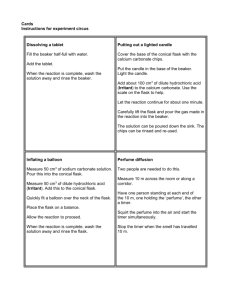
Avogadro and Gay-Lussac’s Laws Kyle Baseden Chemistry I Gas Laws/ Avogadro’s and Gay-Lussac’s Law 2/20/15 CCSS.MATH.CONTENT.HSA.SSE.A.1.A-Interpret parts of an expression, such as terms, factors, and coefficients. 1. Materials 1 250 mL Erlenmeyer flask about 10 mL of water balloon hot plate 2. Objective(s) calculate the change in the number of moles or volume of a substance when one of the factors stay the same. Calculate the change in the pressure or temperature of a gas when one of the factors remains constant throughout the change. Differentiate between these laws and Boyle’s and Charles’ Laws o Recognize when to apply which law to a situation. Be able to convert between grams and moles Convert between pressure units successfully 3. Motivation Engagement o Balloon in a flask At the beginning of the lesson, students will see a balloon that is sucked into a flask. To get the balloon into the flask o Take one 250 mL Erlenmeyer flask and fill with a small (about 10 mL) amount of water o Bring the water to a boil on a hot plate o Quickly cover the mouth of the flask with a balloon while the water is boiling. o Remove the flask from the hot plate and allow to cool o The balloon should be sucked into the flask If this does not happen, the balloon may need to be guided into the flask. The instructor should ask the students if they have any idea how this could happen using any knowledge they have. The reason this does happen is because as the temperature of the gas decreases the volume the gas occupies decreases, so the pressure from the atmosphere pushes on the balloon forcing it into the flask. 4. Goal for Learner Students should be able to recognize the difference between these two laws and the gas laws taught in the previous lesson while also being able to accurately apply Avogadro’s and Gay-Lussac’s laws in problems. Revised 4/11 5. Content and Procedures The instructor will begin the lesson by having a short discussion over the demonstration in the engagement section. The instructor will then begin the lesson by giving a lecture over Avogadro’s and GayLussac’s laws. o Avogadro’s Law You should remember that Avogadro is most remembered for his work in defining the number of molecules/atoms/formula units in a mole. He also did work with gases. He determined that under constant pressure and temperature, that the number of moles of a gas is directly correlated to the volume of a gas. His law is summarized in the following equation: o V1/n1 = V2/n2 o Working with Avogadro’s Law Example If the volume of 25.0 g of F2 is 15.4 L, what volume would the gas have if the F2 was decreased to 15.0 g? Example If the volume of 30.0 g of methane (CH4) is decreased from 13.2 L to 9.8 L was amount of mass was lost? o Gay-Lussac’s Law Gay-Lussac determined the relationship between the temperature and pressure of a gas. He determined that the pressure and temperature of a gas are directly related. The instructor should ask the students what they think the equation will work on will look like. His law can be represented mathematically by the following equation: o P1/T1 = P2/T2 Remember that the temperature has to be in Kelvin when using this equation. o Using Gay-Lussac’s Law Example What is the pressure of a gas that was originally at 1 atmosphere is increased from 39 degrees Celsius to 100 degrees Celsius. After going over the notes for this lesson students will be advised that an additional review over manometer problems will be given o This was because most of the students seemed to have difficulties after learning the material the first time. The instructor should give a quick review of the material before the class ends. To solve a manometer problem, the first step is to draw a picture of the problem. o Remember that a manometer is a U shaped tube filled with mercury. Revised 4/11 It is probably best to convert all of the pressure units to mmHg as the height difference will be equal to the pressure difference between the objects in mmHg. o You just have to decide whether the pressures should be added up or subtracted from one another. To determine this you need to determine whether the pressure you are solving for has a lower or higher mercury level than the known side. The side with the lower mercury level has the higher pressure. o This is because more force is being pushed on the surface of the mercury forcing it down the tube and up the other side. 6. Practice/Application Students will complete a worksheet over using Avogadro’s and Gay-Lussac’s laws o The worksheet will also cover a review of Boyle’s and Charles’ laws Students will have to differentiate between the possible correlations between factors using gas laws. 7. Evaluation of student learning The instructor should make use of both formal and informal evaluations to gauge student learning throughout the lesson. o Formal The worksheets students are to complete will be collected and graded for correctness. Based on the results from the worksheets, adjustments will be made if necessary. o Informal The instructor should make use of probing questions to continuously monitor and assess student understanding of material presented in this lesson. 8. Closure At the end of the lesson, and before students begin to work on their assigned worksheet o The instructor should be sure to note how the balloon got inside the flask. o Before telling students however, the instructor should have students try to explain how the balloon got inside the flask using knowledge they should have gained through the past two lessons over gas laws. The reason this does happen is because as the temperature of the gas decreases the volume the gas occupies decreases, so the pressure from the atmosphere pushes on the balloon forcing it into the flask. NOTE: This lesson was modified by giving a review over manometer problems. This was done due to the fact that many students struggled with answering problems involving a manometer correctly. Revised 4/11