Virtual Electrochemistry lab
advertisement

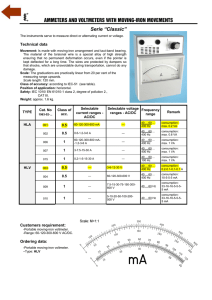
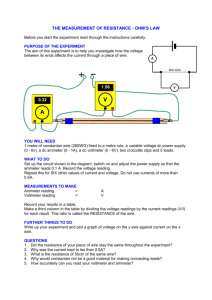
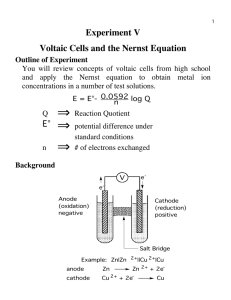
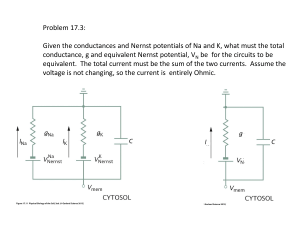
AP Chemistry: Galvanic Cell Virtual Lab Resources: Please go to the following website to complete the tasks for this virtual lab: http://group.chem.iastate.edu/Greenbowe/sections/projectfolder/flashfiles/electroChem/voltai cCellEM F.html In your laboratory notebook, provide a schematic of each cell, labeling the following: 1. Anode, cathode, direction of the flow of electrons, half-reactions at each electrode and flow of ions through the salt bridge, and reading on the voltmeter. 2. For each cell, provide the calculations used to verify the voltage displayed by the virtual voltmeter. Tasks to complete: 1) Create the common Zn/Cu Galvanic Cell under standard conditions. 2) Create conditions where a single replacement reaction occurs spontaneously in only one of the half cells. Explain why no voltage is displayed by the voltmeter. 3) Create two cells under standard conditions, one having the highest and one having the lowest voltage potential possible with the given materials in the virtual lab. 4) Create a concentration cell using any of the metals provided. 5) Using any of your standard cells from above, create a cell under nonstandard conditions that a increases in voltage from the standard cell b decreases in voltage from the standard cell c Does not change the voltage from the standard cell. Conclusion Questions for AP Chemistry: Galvanic Cell Virtual Lab 1. If the leads on the volt meter are reversed (ie. you swap the positions of the electrodes) what happens to the sign on the voltage? Do the electrons still flow FATCAT? Explain. 2. Calculate G for the cell you created in Question 1. 3. What are the values for standard conditions and why did scientists choose these values? 4. What is the Nernst equation? Why would you need to use it? 5. What is the reaction Quotient and how do you calculate it? 6. Explain which R constant is used in the Nernst equation and why you would use this one rather than R from the ideal gas law. 7. Use the Nernst equation to Calculate Ecell for the cell you created in #4. Remember is one has nonstandard conditions. a. You will first need to find the reaction quotient. What are the products and reactants for the reaction? b. How would you find Ecell? c. Plug your numbers into the Nernst equation and find Ecell for the concentration cell. 8. Predict the effect that running the first cell you created in a walk in refrigerator, temperature = 4C, would have on Ecell. You do not have to do an actual calculation. Just explain your prediction using the Nernst equation.