AP Chemistry Lab 2 What is the Relationship Between the
advertisement

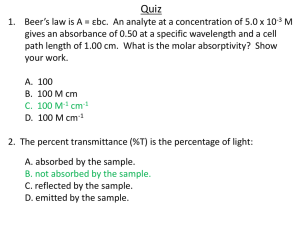
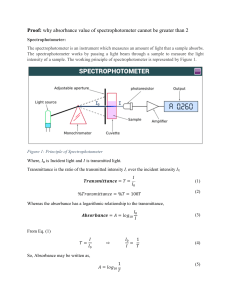
AP Chemistry Lab 2 What is the Relationship Between the Concentration of a Solution and the Amount of Transmitted Light Though the Solution? (Investigation 1; AP Chemistry Guided Inquiry Laboratories, 2013) Much of the experimental work of this lab was completed as the “Prelab Guiding Questions/Simulations”. The experimental portion using the Gatorade happened quickly, and you may have missed it! If so, make sure you get the data from someone who has it. (Adam or Ben?) In your lab notebook: (to be collected around Nov 1, to be part of mid-trimester assessment) For each experiment, starting on a new page, you should have a heading including: title, date(s) of experiment, lab partners Following that should be notes about the lab, observations, labeled data tables, calculations, and hand-written responses to questions asked along the way during the lab. Sketches of particular lab set-ups or explanations about specialized devices (like spectrophotometers) are also appropriate. Remember, the lab notebook is your record of what you did, how you did it, and what you observed and measured. If you refer back to it in 6 months or a year, there should be enough information to clearly jog your memory. And for someone else reviewing it, they should understand the scope of what you did. In your written (word-processed) report: (due 10/21) Introduction: Discussion of concepts and context: spectroscopy, transmittance, absorbance and purpose(s) of lab (both in terms of determining relationship between transmittance, absorbance and concentration, and in terms of your experimental findings about Gatorade.) Prelab: (25 points) Summary of your procedure Table of class data Calculations to transform Transmittance Graphs of each proposed relationship (labeled, titled, with linear regression and correlation values) Your interpretation/assessment of those graphs. (i.e. – what do they tell you about the relationship between Transmittance and concentration?) Essentially, you are developing the concept of Absorbance here. Absorbance = _________. I know this because …….. Investigation: (13 points) Summary of procedure Data Calculations: Absorbance Concentration Mass of blue dye #1 in 500 mL bottle Responses to Post-lab Assessment questions 1-3 (12 points)