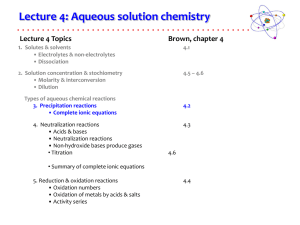
Solution Lab Handout
advertisement
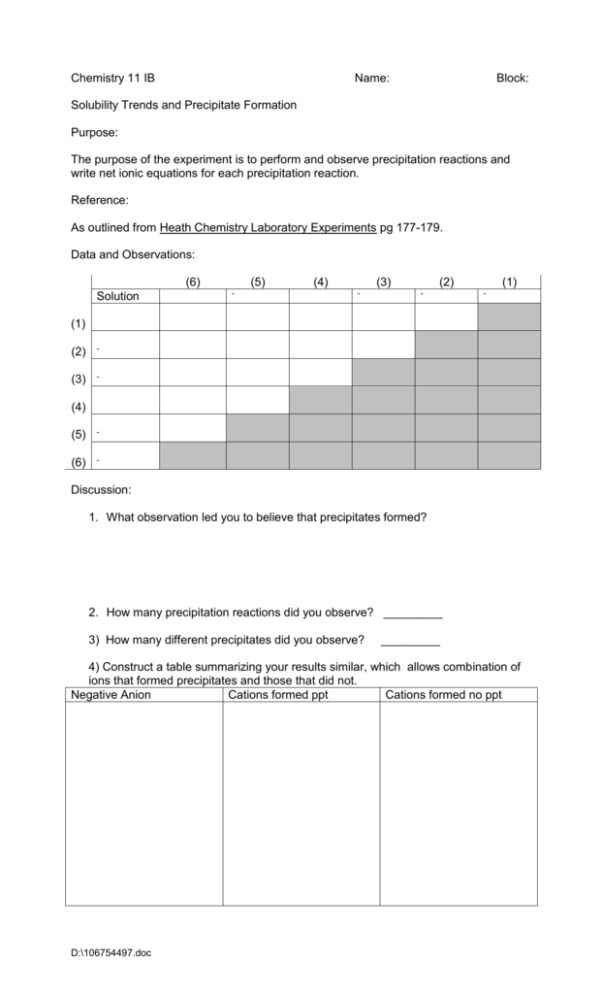
Chemistry 11 IB Name: Block: Solubility Trends and Precipitate Formation Purpose: The purpose of the experiment is to perform and observe precipitation reactions and write net ionic equations for each precipitation reaction. Reference: As outlined from Heath Chemistry Laboratory Experiments pg 177-179. Data and Observations: (6) Solution (5) - (4) (3) - (2) - (1) - (1) (2) - (3) - (4) (5) - (6) - Discussion: 1. What observation led you to believe that precipitates formed? 2. How many precipitation reactions did you observe? _________ 3) How many different precipitates did you observe? _________ 4) Construct a table summarizing your results similar, which allows combination of ions that formed precipitates and those that did not. Negative Anion Cations formed ppt Cations formed no ppt D:\106754497.doc 5) For each different precipitation, write the following: a) balanced chemical equation b) complete ionic equation c) net ionic equation 6) Compare your table from question 4 above to the solubility table located in the data booklet. Describe any differences or similarities in results. Conclusion: What did you learn from the experiment? D:\106754497.doc