bonding workbook version1
advertisement
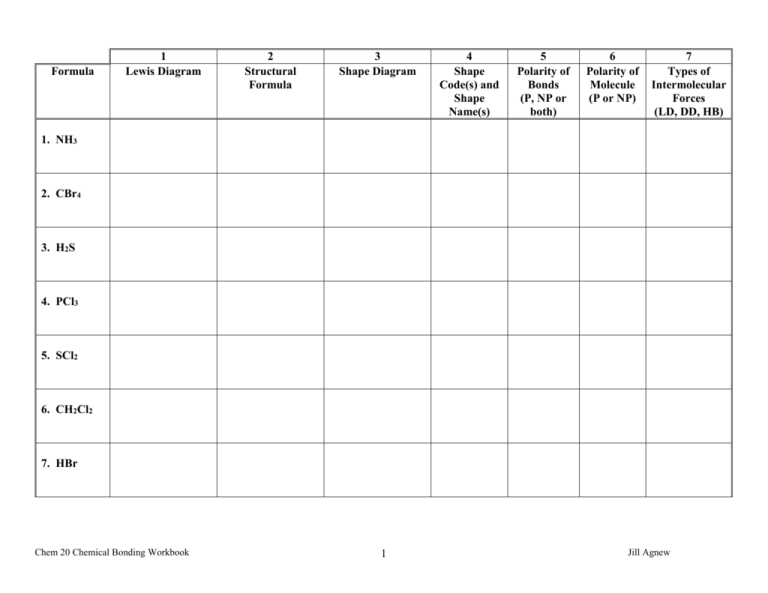
Formula 1 Lewis Diagram 2 Structural Formula 3 Shape Diagram 4 Shape Code(s) and Shape Name(s) 5 Polarity of Bonds (P, NP or both) 6 Polarity of Molecule (P or NP) 7 Types of Intermolecular Forces (LD, DD, HB) 1. NH3 2. CBr4 3. H2S 4. PCl3 5. SCl2 6. CH2Cl2 7. HBr Chem 20 Chemical Bonding Workbook 1 Jill Agnew Formula 1 Lewis Diagram 2 Structural Formula 3 Shape Diagram 4 Shape Code(s) and Shape Name(s) 5 Polarity of Bonds (P, NP or both) 6 Polarity of Molecule (P or NP) 7 Types of Intermolecular Forces (LD, DD, HB) 8. OCl2 9. NI3 10. O2 11. SBr2 12. HCl 13. H2Te 14. NF3 Chem 20 Chemical Bonding Workbook 2 Jill Agnew Formula 1 Lewis Diagram 2 Structural Formula 3 Shape Diagram 4 Shape Code(s) and Shape Name(s) 5 Polarity of Bonds (P, NP or both) 6 Polarity of Molecule (P or NP) 7 Types of Intermolecular Forces (LD, DD, HB) 15. H2Se 16. CH3OH 17. CHCl3 18. Cl2 19. CS2 20. CH4 21. OF2 Chem 20 Chemical Bonding Workbook 3 Jill Agnew Formula 1 Lewis Diagram 2 Structural Formula 3 Shape Diagram 4 Shape Code(s) and Shape(s) 5 Polarity of Bonds (P, NP or both) 6 Polarity of Molecule (P or NP) 7 Types of Intermolecular Forces (LD, DD, HB) 22. C2H2 23. CO2 24. NCl3 25. C2H4 26. H2O 27. SiH4 28. CHCF Chem 20 Chemical Bonding Workbook 4 Jill Agnew Boiling Points and Number of Electrons for Groups 14-17 Hydrogen Compounds Group Compound CH4 14 15 16 17 # of Electrons Boiling Point 162C SiH4 112C GeH4 90C SnH4 50C NH3 33C PH3 87C AsH3 60C SbH3 25C H2O 100C H2S 65C H2Se 45C H2Te 15C HF 20C HCl 85C HBr 69C HI 35C 1. Complete the “Number of Electrons” column. 2. Graph the data above on one set of axes. Label both axes and give it a title. The manipulated variable (# of electrons) goes on the x-axis and the responding variable (boiling point) goes on the y-axis. Join the points for each group separately with a straight line between each point. 3. Label each point on the graph with the compound formula. 4. The hydrogen compounds of Groups 15, 16 and 17 elements have consistently increasing van der Waals forces (except for the first hydrogen compounds) with increasing number of electrons. Explain why the first hydrogen compounds show a reversal in this trend. 5. Explain why CH4 does not show this same reversal in trend that is displayed by the first hydrogen compounds in the other Groups. 6. Explain why the boiling points of the hydrogen compounds of the Group 14 elements are consistently lower than the boiling points of the other hydrogen compounds. Chem 20 Chemical Bonding Workbook 5 Jill Agnew Molecular Compound Number of Electrons Boiling Point (C) 1. F2(g) 188 2. Cl2(g) 35 3. Br2(l) 59 4. I2(s) 84 5. ClF(g) 101 6. BrF(g) 20 7. BrCl(g) 5 8. ICl(g) 97 9. IBr(g) 116 10. CH4(g) 162 11. C2H6(g) 87 12. C3H8(g) 45 13. C4H10(g) 0.50 14. C5H12(l) 36 15. CF4(g) 129 16. CCl4(l) 77 17. CBr4(s) 189 18. CH3F(g) 78 19. CH3Cl(g) 24 20. CH3Br(g) 3.6 21. CH3I(l) 43 22. CH3OH(l) 65 23. C2H5F(g) 38 24. C2H5Cl(g) 13 25. C2H5Br(l) 38 26. C2H5I(l) 72 27. C2H5OH(l) 78 1. Complete the chart above. Chem 20 Chemical Bonding Workbook 6 Jill Agnew Types of Intermolecular Forces London DipoleHydrogen Dispersion Dipole Bonding 2. Explain why there is a difference in boiling point between Br2(g) and ICl(g). 3. Explain why there is a difference in boiling point between BrF(g) and CH3F(g). 4. Why would boiling point increase if number of electrons increases? 5. Explain why methanol (CH3OH(l)) and ethanol (C2H5OH(l)) each have the lowest number of electrons but the highest boiling point of their respective series. 6. Explain why there is a difference in boiling point between Cl2(g) and C4H10(g). 7. Explain why there is a difference in boiling point between BrCl(g) and C2H5Br(l). Chem 20 Chemical Bonding Workbook 7 Jill Agnew Chemical Bonding Review 1. Define the following terms: a) valence level b) electronegativity c) ionic bond d) metallic bond e) covalent bond f) lone pair g) bonding electron h) bonding capacity i) crystal lattice j) nonpolar covalent bond k) polar covalent bond l) polarity m) intermolecular forces n) intramolecular forces o) dipole-dipole forces p) hydrogen bonding q) London Dispersion forces 2. Draw the electron dot diagrams for the following ionic compounds: a) AlCl3 c) magnesium chloride b) Na2S d) calcium phosphide 3. How does electronegativity change down group and across period? 4. What two things affect electronegativity? 5. Compare the properties of ionic compound to the properties of molecular compounds. 6. Draw the Lewis structure for the following: e) PCl3 f) N2 g) CO2 h) SiH4 i) CH3OH j) HCN k) HNS l) C2F4 m) HF n) H2S o) CI4 p) NF3 7. State the shape code and the shape for each of the molecules in question 6. 8. State the polarity of each molecule in question 6. 9. State the intermolecular attractions present for each of the molecules in question 6. Chem 20 Chemical Bonding Workbook 8 Jill Agnew 10.Draw the bond dipole diagram for each of the following bonds and state whether it is polar, nonpolar or ionic: a) H – Br e) H – N b) S – S f) O – Cl c) Ca – I g) F – F d) C – O h) C – H 11.Draw and label a “Scale of Forces” diagram. 12.Explain how conductivity relates to ionic compounds, metals and molecular compounds. Chem 20 Chemical Bonding Workbook 9 Jill Agnew