AP Chem Midterm Review 1213
advertisement

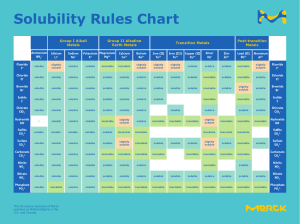
AP Chemistry Midterm Review with selected problems from the end of each chapter for review CHAPTER 13 Miscibility of organic compounds in water P. 518-19 # 15-18 Generalizations about solubility of solids, liquids and gases P. 518-19 # 13-22 Recognizing changes in the entropy of a system Read P. 489-90 Intermolecular forces in compounds P. 443 # 21 Identifying soluble substances P. 518 # 14-17 Solvation Read section 13.1 Like dissolves like P. 519 # 18 Solutions, suspensions and colloids Read P. 511-12 Saturated solutions and solubility Read section 13.2 Colligative properties generalizations P. 502 What happens in freezing point depression P. 506 Factors affecting rates of dissolving Chapter 13 CHAPTER 14 Relationship of temperature to reaction rate P. 568 # 39 Role of a catalyst in rate of reaction P. 569 # 63 Determining the order of reaction P. 565-66 # 13-26 Rate constant units P. 565-66 # 13-26 Writing the rate law expression for a reaction P. 565-66 # 13-26 Energy diagram and activation energy P. 568 # 43-44, sample exercise 14.10 (P. 547) Rate law and the slow step of a mechanism P. 569 # 59-62 Mechanism vs. overall reaction P. 569 # 59-62 Selected problem solving P. 565-66 # 13-26 CHAPTER 15 Writing an equilibrium constant expression P. 605 # 7-8 LeChatelier’s principle P. 607 # 43-46 1 CHAPTER 16 Arrhenius, Bronstead-Lowry and Lewis acids and bases Read sections 16.1, 16.2, 16.11 Characteristics of strong/weak acids and bases Read sections 16.5, 16.6, 16.7 Conjugate acid-base pairs P. 653 # 5-6 Acidic/basic salts P. 656-57 # 75-76 Determining pH P. 654-55 # 27-28 Selected problem solving P. 656 # 56, 65-68 CHAPTER 17 Determining what can be in a buffer system P. 697 # 11-12 Recognizing titration curves: SA/SB, WA/SB, SA/WB, Read section 17.3 WA/WB Writing solubility product constant expressions P. 698 # 37-43 Selected problem solving P. 698 # 23-34 ABOUT THE EXAM Format: Part 1: 40 multiple choice questions (1 point each) Part 2: 2 problems (choose from 3) (15 points each) 1 kinetics 1 acid-base equilibria problem, choose from 2 You will be given a periodic table, AP Exam sheet of formulae and constants, the activity series and the solubility rules with your exam Don’t forget to bring a #2 lead pencil and a calculator to the exam. No cell phones, please! THE GRADING PROCEDURE FOR THE PROBLEMS The questions are from old AP Exams and the established grading criteria will be used. Partial credit will be given in the same manner used during the year. 2 STUDYING SUGGESTIONS 1. Complete the problems suggested in this review packet 2. Check your answers using the chapter answer keys you already have 3. Review your notes for those topics and skills for the problems/questions you had difficulty with 4. Ask questions during the midterm review in class 3