half-life worksheet
advertisement

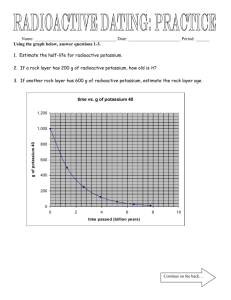
HALF-LIFE WORKSHEET Use the Reference Table to the right to assist you in answering questions 1-3, 5 and 11. 1. How long does it take a 100.00 g sample of As-81 to decay to 12.5 g? ½ life: As-81 = 33 seconds 2. How long does it take a 180 g sample of C-14 to decay to ⅛ its original mass? Au-198 = 2.69 days C-14 = 5730 years 3. What percent of a sample of As-81 remains un-decayed after 66 seconds? 4. What is the half-life of a radioactive isotope if a 500.0 g sample decays to 62.5 g in 39.3 hours? 5. How old is a bone if it presently contains 0.3125 g of C-14, but it was estimated to have originally contained 80.000 g of C-14? 6. What is the half-life of a 100.0 g sample of nitrogen-16 that decays to 12.5 g of nitrogen-16 in 21.6 s? 7. All isotopes of technetium are radioactive, but they have widely varying half-lives. If an 800.0 g sample of technetium-99 decays to 50.0 g of technetium-99 in 852,000 yr, what is its half-life? 8. Thallium-208 has a half-life of 3.053 min. How long will it take for 120.0 g to decay to 7.50 g? 9. If the half-life of iodine-131 is 8.10 days, how long will it take a 50.00 g sample to decay to 6.25 g? 10. Potassium-42 has a half-life of 12.4 hours. How much of an 848 g sample will be left after 62.0 h? 11. How much of a 144 g sample of carbon-14 will remain after 17,190 yr? 12. Use the bar graph to the left to answer a-c. 100% Amount of Chromium -48 a. How long is a half-life for chromium-48? 75% b. If only 25% of the chromium-48 remains, how old is the material containing the chromium-48? 50% 25% 12.5% 0 21.6 43.2 64.8 Time (h) c. If a sample originally had 120 atoms of chromium-48, how many atoms will remain after 64.8 hours? 13. If the half-life of uranium-235 is 7.04 × 108 yr and 12.5 g of uranium-235 remain after 28.16 × 108 yr, how much of the radioactive isotope was in the original sample? 14. A 208 g sample of sodium-24 decays to 13.0 g of sodium-24 within 60.0 h. What is the half-life of this radioactive isotope? HALF-LIFE PROBLEMS 1) The half-life of Zn-71 is 2.4 minutes. If one had 100.0 g at the beginning, how many grams would be left after 7.2 minutes has elapsed? 2) Pd-100 has a half-life of 3.6 days. If one had 6.02 x 1023 atoms at the start, how many atoms would be present after 18.0 days? 3) Os-182 has a half-life of 21.5 hours. What fraction and how many grams of a 10.0 gram sample would have decayed after exactly three half-lives? 4) After 24.0 days, 2.00 milligrams of an original 128.0 milligram sample remain. What is the half-life of the sample? 5) U-238 has a half-life of 4.46 x 109 years. How much U-238 should be present in a sample 8.92 x 109 years old, if 2.00 grams was present initially? 6) How long will it take for a 40.0 gram sample of I-131 (half-life = 8.040 days) to decay to 1/32 its original mass? 7) Fermium-253 has a half-life of 0.334 seconds. A radioactive sample is considered to be completely decayed after 5 half-lives. How much time will elapse for this sample to be considered gone? 8) At time zero, there are 10.0 grams of W-187. If the half-life is 24.0 hours, how much will be present at the end of 4 days (96 hours)? 9) 100.0 grams of an isotope with a half-life of 36.0 hours is present at time zero. How much time will have elapsed when 12.5 grams remains? 10) How much time will be required for a sample of H-3 to lose 75% of its radioactivity? The half-life of tritium is 12.26 years. 11) The half life of iodine-131 is 8.040 days. What percentage of an iodine-131 sample will remain after 40.2 days? 12) The half-life of thorium-227 is 18.72 days. How many days are required for three-fourths of a given amount to decay? 13) If you start with 5.32 x 109 atoms of Cs-137, how much time will pass before the amount remaining is 3.33 x 108 atoms (0.333 x 109 atoms)? The half-life of Cs-137 is 30.17 years. 14) The half-life of the radioactive isotope phosphorus-32 is 14.3 days. How long until a sample loses 50% of its radioactivity? ANSWERS 1) 12.5 g 3) ⅛ and 1.25 g 7) 1.67 s 11) 3.125 % 2) 0.188 x 1023 or 1.88 x 1022 atoms 4) 4 days 5) 0.50 g 8) 0.625 g 9) 108.0 h 12) 37.44 days 13) 120.68 yr 6) 48.24 days 10) 24.52 yr 14) 14.3 days