姓名?學號?關鍵字
每篇文章(資料)的作者、篇名、出處等資料
何謂炭疽熱?
炭疽熱是因感染到一種稱為 Bacillus anthracis 的細菌所引發的急性病, 大都發生
在一些草食動物, 如牛, 羊等, 人類如果接觸到含這種菌孢(spore)的動物及其排
泄物, 也會發病.
因此這個病大都出現在農村, 農場及草食動物的牧場. 中南美, 東歐, 亞洲, 非
洲, 加勒比海小島等則是較常發現這個病的地區.
Bacillus Anthracic 是一種 Gram(+)桿菌, 大小約 1-1.5um X 4-10um, 其孢子體可以
在乾燥的狀態下存活數年之久,因此, 很容易被傳遞而利用為一種生物戰劑.
但是煮沸 10 分鐘就可以把它全部破壞, 另外,碘酒, 雙氧水, 福馬林,甚至洗潔精
也可以有效的消除之.
經由什麼途徑感染人體? 各有何症狀?
(一)經由皮膚
Bacillus anthracis 的菌孢(spore)可以經由受傷的皮膚傷口侵入, 也會經由帶菌
孢的昆蟲咬傷後侵入, 受侵入的皮膚, 經過 4-7 天的潛伏, 首先會有一個腫起
的環狀紅斑, 1-2 天之後變成無痛的水泡, 然後潰瘍. 中心呈黑色的結痂, 周
圍則呈棕色的腫起, 80-90%的病人會自然痊癒, 但要數個星期之久. 但是有
10-20%的病人, 會轉變成全身菌血症, 高燒並快速的因溶血而死亡.
(二)經由吸入
Bacillus anthracis 的菌孢(spore)漂浮在空氣中, 經由呼吸而吸入肺部, 只需 1-3
天就病發, 剛開始很像一般感冒,隨即很快的就進入急性狀態, 發高燒, 呼吸
短促, 呼吸困難, 缺氧, 休克, 並大都(90%)在 24 小時內死亡, 因為進展很快,
很不容易在第一時間就診斷得出來.
(三)經由飲食
含有 Bacillus anthracis 的菌孢(spore), 可以經由飲食而進入胃腸, 引起急性腸
炎, 導致發燒, 噁心, 嘔吐,帶血性下痢, 腹水,約 25-60%的死亡率.
如果菌孢從扁桃腺侵入, 則會引起喉嚨痛,吞嚥困難,發燒, 頸部淋巴腺疼痛,
呼吸困難等症狀.
會不會人與人之間的傳染?
並不會人與人之間的直接傳染, 即使是吸入性炭疽熱的病人, 也不會經由他的呼
吸, 再經空氣而感染至其他人.
如何診斷與預防?
(一)如何診斷?
Direct Gram's staining of the skin lesion
Direct fluorescent antibody staining
Direct skin lesion culture , or blood culture(但是常常在培養細菌的結果還沒出來
以前, 病人就已死亡了)
Tests for antibody to Bacillus anthracis 是比較快速的診斷方法.
(二)如何預防?
1.進入炭疽熱常見的地區時, 盡量避免接觸到家畜動物,其產物及排泄物, 食
物應熱食.
2.預防注射
The anthrax vaccine 是由 BioPort, Corporation, Lansing, Michigan 製造, 預防效
果可以高達 93%, 這疫苗是經由皮下注射, 每兩星期打一次, 連續打三次之
後再於第 6, 12, 18 個月各施打一次, 以後還需每年注射一次.
如何治療?
對 Gram(+)有效的抗生素都可以用來治療炭疽熱, 最有效的當首推 Penicillin G.
剛開始以 200 萬單位每 6 小時注射一次, 直至症狀消退, 再以口服 penicillin 7-10
天. 對 penicillin 過敏的病人, 則給予 ciprofloxacin, erythromycin, tetracycline, 或
chloramphenicol.
*皮膚炭疽熱還必須清理傷口並預防污染其他物品.
*吸入性炭疽熱則必須給予更大量的抗生素, Penicillin G.應以每兩小時 200 萬單
的劑量注射.
http://www.lai-obs.com/page7d.htm 出處
賴國良….作者
Bacillus anthracis
From Wikipedia, the free encyclopedia
Jump to: navigation, search
?
Bacillus anthracis
Photomicrograph of Bacillus anthracis
(fuchsin-methylene blue spore stain).
Scientific classification
Kingdom: Bacteria
Phylum:
Firmicutes
Class:
Bacilli
Order:
Bacillales
Family:
Bacillaceae
Genus:
Bacillus
Species:
B. anthracis
Binomial name
Bacillus anthracis
Cohn 1872
Bacillus anthracis is a Gram positive, rod-shaped bacterium of the genus Bacillus. A
spore-forming bacteria, B. anthracis is a natural soil-dwelling organism, as well as the
causative agent of anthrax.
Each cell is about 1 by 6 μms in size.
Historical background
B. anthracis was the first bacterium conclusively demonstrated to cause disease, by Robert
Koch in 1877.
The specific name anthracis comes from the Greek word anthrax (ἄνθραξ),
meaning coal and referring to the most common form of the disease, cutaneous anthrax, in
which large black skin lesions are formed.
Pathogenicity
Under conditions of environmental stress, B. anthracis bacteria naturally produce
endospores which rest in the soil and can survive for decades in this state. When ingested
by a cattle, sheep, or other herbivores, the bacteria begin inside the animal and eventually
kill it, then continue to reproduce in its carcass. Once the nutrients are exhausted, new
endospores are produced and the cycle repeats.
B. anthracis has as least 89 known strains, ranging from highly virulent strains with
biological warfare and bioterrorism applications (Ames and Vollum) to benign strains used
for inoculations (Sterne). The strains differ in presence and activity of various genes,
determining their virulence and production of antigens and toxins.
References
Madigan M; Martinko J (editors). (2005). Brock Biology of Microorganisms,
11th ed., Prentice Hall. ISBN 0131443291.
Ryan KJ; Ray CG (editors) (2004). Sherris Medical Microbiology, 4th ed.,
McGraw Hill. ISBN 0838585299.
Wikipedia® http://en.wikipedia.org/wiki/Bacillus_anthracis 出處
?
編輯
炭疽桿菌
分類
域: 細菌域(Bacteria)
門: 厚壁菌門(Firmicutes)
綱: 芽孢桿菌綱(Bacilli)
目: 芽孢桿菌目(Bacillales)
科: 芽孢桿菌科(Bacillaceae)
屬: 芽孢桿菌屬(Bacillus)
種: 炭疽芽孢桿菌(B. anthracis)
學名
Bacillus anthracis
Cohn 1872
Bacillus Anthracis
Anthrax
Excerpt from Center for civilian biodefense strategies
http://www.hopkins-biodefense.org/pages/agents/agentanthrax.html
Bacillus anthracis, the organism that causes anthrax, derives its name from the
Greek word for coal, anthracis, because of its ability to cause black, coal-like
cutaneous eschars.
Anthrax infection is a disease acquired following contact with infected
animals or contaminated animal products or following the intentional release
of anthrax spores as a biological weapon.
One of the major problems with anthrax spores is the potentially long
incubation period of subsequent infections. Exposure to an aerosol of anthrax
spores could cause symptoms as soon as 2 days after exposure. However,
illness could also develop as late as 6-8 weeks after exposure -- in Sverdlovsk,
one case developed 46 days after exposure.
Excerpt taken from:
http://www.bact.wisc.edu/Bact330/lectureanthrax
The anthrax bacillus, Bacillus anthracis, was the first bacterium shown to be
the cause of a disease. In 1877, Robert Koch grew the organism in pure culture,
demonstrated its ability to form endospores, and produced experimental
anthrax by injecting it into animals.
Bacillus anthracis is very large, Gram-positive, sporeforming rod, 1 - 1.2 痠 in
width x 3 - 5 痠 in length. The bacterium can be cultivated in ordinary nutrient
medium under aerobic or anaerobic conditions. Genotypically and
phenotypically it is very similar to Bacillus cereus, which is found in soil
habitats around the world, and to Bacillus thuringiensis, the pathogen for
larvae of Lepidoptera. The three species have the same cellular size and
morphology and form oval spores located centrally in a nonswollen
sporangium.
Robert Koch's original micrographs of the anthrax bacillus
Bacillus anthracis. Gram stain. The cells have characteristic squared ends. The
endospores are ellipsoidal shaped and located centrally in the sporangium. The spores
are highly refractile to light and resistant to staining.
Cutaneous Anthrax-Vesicle Development
Pictures taken from the CDC website:
http://www.bt.cdc.gov/agent/anthrax/anthrax-images/cutaneous.asp
Day 2
Day 4
Eschar formation
Day 6
Day 10
Day 15
Inhalational Anthrax
Pictures taken from the CDC website:
http://www.bt.cdc.gov/agent/anthrax/anthrax-images/inhalational.asp
Mediastinal widening with inhalational anthrax (JAMA 1999:281:1735-1745)
Mediastinal widening and pleural effusion on Chest X-Ray in inhalational anthrax
Gastrointestinal Anthrax
Pictures taken from the CDC website:
http://www.cdc.gov/ncidod/eid/vol8no7/02-0062.htm
Figure 1. A 29-year-old man, 1 day after the onset of symptoms of oropharyngeal
anthrax. Marked and painful swelling of the right side of the neck was present.
Figure 2. A 27-year-old man, 5 days after the onset of symptoms of oropharyngeal
anthrax. Edema and congestion of the right tonsil extending to the anterior and
posterior pillars of fauces as well as the soft palate and uvula were present. A white
patch had begun to appear at the center of the lesion.
Figure 3. The same patient as in Figure 2. This picture is 9 days after the onset of
symptoms of oropharyngeal anthrax. The white patch had developed into a
pseudomembrane covering the lesion.
Excerpt taken from:
http://www.bact.wisc.edu/Bact330/lectureanthrax
Treatment of Anthrax
Antibiotics should be given to unvaccinated individuals exposed to inhalation
anthrax. Penicillin, tetracyclines and fluoroquinolones are effective if
administered before the onset of lymphatic spread or septicemia, estimated to
be about 24 hours. Antibiotic treatment is also known to lessen the severity of
disease in individuals who acquire anthrax through the skin. Inhalation anthrax
was formerly thought to be nearly 100% fatal despite antibiotic treatment,
particularly if treatment is started after symptoms appear. A recent Army study
resulted in successful treatment of monkeys with antibiotic therapy after being
exposed to anthrax spores. The antibiotic therapy was begun one day after
exposure.
http://www.bt.cdc.gov/agent/anthrax/faq/signs.asp
What specific symptoms should I watch for?
People should watch for the following symptoms:
Fever (temperature greater than 100 degrees F). The fever may be
accompanied by chills or night sweats.
Flu-like symptoms
Cough, usually a non-productive cough, chest discomfort, shortness of breath,
fatigue, muscle aches.
Sore throat, followed by difficulty swallowing, enlarged lymph nodes,
headache, nausea, loss of appetite, abdominal distress, vomiting, or diarrhea.
A sore, especially on your face, arms or hands, that starts as a raised bump and
develops into a painless ulcer with a black area in the center.
Anthrax
(Bacillus
anthracis)
(Released
November
2001)
by Roberta A. Gardner
Overview
Key Citations
Web Sites
Glossary
Conferences
Editor
Overview
Biology
Living in the soil are huge numbers of bacteria belonging to the genus Bacillus. This genus
consists of aerobic, Gram-positive, spore-forming rods. All of the members share certain
characteristics, including the ability to exist in two different forms. When conditions for growth
are good, with plentiful nutrients and water available, they are rod-shaped organisms that grow
and divide. When conditions are unfavorable, each forms a very resistant dormant spore that is
able to survive extreme environmental conditions.
The spore is a dehydrated cell with thick walls and additional layers that form inside the cell
membrane. It can remain inactive for many years, but if it comes into a favorable environment,
it begins to grow again. It is sometimes called an endospore, because it initially develops inside
the rod-shaped form. Features such as the location within the rod, the size and shape of the
endospore, and whether or not it causes the wall of the rod to bulge out are characteristic of
particular species of Bacillus. Depending upon the species, the endospores are round, oval, or
occasionally cylindrical. They are highly refractile and contain dipicolinic acid. Electron
micrograph sections show that they have a thin outer spore coat, a thick spore cortex, and an
inner spore membrane surrounding the spore contents. The spores resist heat, drying, and many
disinfectants (including 95% ethanol).1
Most Bacillus species grow on dead and decaying organic matter, and are harmless to man and
animals. One species, however, Bacillus anthracis, is a dangerous pathogen that causes the
disease anthrax. It is a zoonosis, meaning that it affects domestic and wild animals, and,
secondarily humans.
The ability of Bacillus anthracis to form spores makes it a difficult organism to control. Spores can
exist in the soil for decades. They can drift gently in the wind, dormant until they find a place that
has the temperature, nutrients, and other conditions to allow growth. When they find their new
host (an animal or human) they change to the rod-like form and begin to multiply rapidly. While
they are in the spore form they can survive boiling, freezing, or even suspension in alcohol. It
takes special measures to kill them, such as steam under pressure, or chemicals known as
sporicides. This ability to survive extreme conditions for long periods of time is one of the major
reasons Bacillus anthracis has been used by terrorists.
For a microbiologist, growing Bacillus anthracis in the laboratory and causing it to form spores is
an easy task. However, putting a culture containing millions of Bacillus anthracis spores into a
form that makes an effective weapon is not easy.
Disease
Bacillus anthracis is typically a disease of herbivores (plant-eating mammals), although it can
affect other animals as well. Among domestic animals, cattle, sheep, and goats have been the
most frequent victims. In most industrialized countries, livestock are routinely vaccinated, and
cases of anthrax are rare. In developing countries, however, where animal vaccination is not
regularly practiced, anthrax in animals is a problem. This is especially so in tropical and
sub-tropical environments. In the USA, anthrax cases among animals have been generally
limited to the western plains.
Endospores can survive in the soil for years. Animals consume the spores along with grass as they
graze. After the spores enter an animal, they germinate, changing from the resistant form into
the growing and dividing vegetative form. The sporangium lyses, the spore germinates, and the
bacilli multiply rapidly.
Anthrax is a very serious disease in animals, culminating in a fatal septicemia. The carcass of the
animal should be burned where it lies. The carcass should never be opened, since doing this will
cause the vegetative forms, which can be destroyed relatively easily, to form into the resistant
spores that can survive for years.
Virulent Bacillus anthracis vegetative cells form capsules of poly-D-glutamic acid after they enter
their host. The capsule has a negative charge which inhibits macrophages from engulfing and
destroying the vegetative cells, impeding the hosts immune response. Thus the capsule allows
virulent anthrax bacilli to grow virtually unimpeded in the infected host.2
Infection in humans traditionally has been much rarer than infection in animals. Anthrax occurred
in people who came in contact with animals or animal products. It was frequently an occupational
disease, affecting veterinarians, people who raised livestock, and people who prepared products
from wool, hide, and hair of animals. The inhalation form of anthrax used to be known as
woolsorters disease, because it affected people who worked with wool. Products from countries
where anthrax in animals is prevalent are still a problem. Goat hair and handicrafts containing
animal hides from the Middle East have been a repeated source of infection. 3 The ordinary citizen
in the USA is most likely to encounter anthrax from imported products that have not been treated
sufficiently to destroy spores.
In humans there are three possible forms of the disease anthrax. Historically, the most common
form has been cutaneous anthrax, in which the organism enters through a break in the skin. The
cutaneous form begins as a papule at the entry site that progresses over several days to a vesicle
and then ulcerates. Edema, sometimes massive, surrounds the lesions, which then develop a
characteristic black eschar. The patient may have fever, malaise and headache.4 A small
percentage of cutaneous infections become systemic, and these can be fatal.
A more serious form is inhalation anthrax. Here the victim breathes in the organism and develops
a severe respiratory disease. Systemic infection resulting from inhalation of Bacillus anthracis has
a mortality rate approaching 100%. Initial symptoms are vague and flu-like, progressing to
hypotension, shock and massive bacteremia and toxemia. The severe symptoms are believed to
be the result of the bacillis exotoxins. Early antibiotic treatment is an absolute necessity and
should be started during the incubation period if a person has been exposed.5 After acute
symptoms have appeared, antibiotics can kill the organisms, but will not destroy the powerful
toxins that have already been formed, and the person commonly dies in 2-3 days from
respiratory failure, sepsis and shock.
The third form, intestinal anthrax, is contracted from the consumption of contaminated meat. In
industrialized countries this is not usually a risk, although rare exceptions have been described.
In August 2000, the Minnesota Department of Health was notified that Bacillus anthracis had
been isolated from a steer on a farm in Roseau County. The infected steer was one of five dead
cattle found in a pasture. On the basis of identification of the bacteria by phage typing of isolates
cultured from tissues and blood samples by the North Dakota State University Veterinary
Diagnostic Laboratory, anthrax was confirmed. A report of this incident described the
management of and public health response to human exposure to meat contaminated with
anthrax.6
In countries where hunger is a serious problem, ingestion of contaminated meat is more of a risk
than it is in the U.S. Oropharyngeal anthrax begins with severe sore throat or with an ulcer in the
oropharyngeal cavity, accompanied by neck swelling and fever. Gastrointestinal anthrax begins
with anorexia, nausea, vomiting and abdominal pain. There may be hemorrhagic diarrhea.7
Intestinal anthrax can become systemic and lead to death.
Anthrax can be treated with antibiotics, but it is essential that treatment begin early. If it is known
that a person has been exposed, treatment should begin immediately, even before symptoms
appear. In inhalation anthrax, the most serious form of the disease, the initial symptoms are
general flu-like, respiratory symptoms. If treatment is delayed until specific symptoms appear,
the fatality rate is extremely high. Ciprofloxacin and doxycycline are the drugs of choice. Penicillin
can also be used. Because it is essential to completely eradicate the organism, treatment of
anthrax must continue for an extended period, generally sixty days.
Because of current public health concerns, The New England Journal of Medicine has published
three articles on the Web at www.nejm.org, several weeks prior to their appearance in print in the
November 29, 2001, issue.
"Cutaneous anthrax infection" (published Nov. 6) by K.J. Roche, M.W. Chang and H. Lazarus
describes the case of a seven-month old male infant who was hospitalized with a two-day history
of swelling of the left arm and a weeping lesion at the left elbow. It was first diagnosed as a spider
bite. He was treated with ampicillin-sulbactam and clindamycin. He had been at his mothers
office at a television network three days before admission. After anthrax exposure was reported
at another television network, two punch biopsies of the lesion were performed. Polymerase
chain reaction and immunostaining for Bacillus anthracis were positive.
"Recognition and management of anthraxan update" (published Nov. 6) by M.N. Swarz reviews
the characteristics of the organism and the diagnosis and treatment of the disease.
"Index case of fatal inhalational anthrax due to bioterrorism in the United States" (published Nov.
8) by L.M. Blush, B.H. Abrams, A. Beall, and C.C. Johnson describes in detail the first case of
inhalation anthrax to occur in the United States since 1978. The patient was employed as a photo
editor for a major tabloid newspaper in Florida, where he spent most of the day reviewing
photographs submitted by mail or over the Internet. Coworkers report that the patient had
closely examined a suspicious letter containing powder approximately eight days before the
onset of illness. Bacillus anthracis was cultured from blood and from cerebrospinal fluid.
Research
It has been known for years that the part of the organism that causes the symptoms of disease
is anthrax toxin, a very powerful poison. Much of the current research on anthrax involves
examining the toxin in great detail, making small changes in its structure, and seeing how the
changes affect its properties. Researchers are trying to understand every step in how the toxin
exerts its effects. If the procedure is thoroughly understood, then researchers can look for
specific steps where they can block the actions of the toxin. A recent article in Critical Reviews in
Microbiology (vol. 27, no. 3, pp. 167-200) by R. Bhainagar and S. Batra reviews anthrax toxin.
Low levels of anthrax toxin induce release of cytokines such as tumor necrosis factor alpha.
Dehydroepiandrosterone and melatonin have been found to inhibit the increased cytokine
production of the anthrax lethal toxin and may have a role in therapy.8
It has been found that anthrax toxin actually consists of three proteins. Protective antigen (PA)
binds to a cell receptor and mediates the entry of the other two components to the cytoplasm.
Edema factor (EF), named for its ability to produce edema, is a calcium/calmodulin-dependent
adenylate cyclase. Lethal factor (LF), the dominant virulence factor associated with the toxin,
proteolytically inactivates mitogen-activated protein kinase kinases (MAP kinase kinases), which
are important in intracellular signal transduction.9
The three separate proteins that make up anthrax toxin PA, EF and LF act in binary combinations
to produce two distinct reactions in experimental animals: edema (PA+EF) and death (PA+LF).10
There has been a great deal of interest in PA, since it is essential for the activity of both EF and LF.
It has been found that a proteolytically activated 63-kDa fragment of PA binds LF/EF and
translocates them into the cytosol of mammalian cells. Domain II of PA has been implicated in
membrane insertion and channel formation.
One study found that a PA mutant had considerably reduced toxicity in combination with LF, as
well as decreased membrane insertion and translocation of LF into the cytosol.11 In another study,
mutations blocked the ability of PA to mediate pore formation and translocation in cells but had no
effect on its receptor binding, proteolytic activation, or ability to oligomerize and bind the toxin's
enzymes.12 Another study identified mutants of PA that co-assemble with the wild-type protein
and block its ability to translocate the enzymes across membranes. These mutants strongly
inhibited toxin action in cell culture and in an animal intoxication model, suggesting they could be
useful in therapy of anthrax.13
PA and LF acting together to produce death in animals are often referred to as lethal toxin. It has
been shown that lethal toxin suppresses proinflammatory cytokine production in macrophages by
inhibiting transcription of cytokine messenger RNA, even at extremely low levels of lethal toxin.
Thus, one way lethal toxin causes the disease anthrax is by suppressing the inflammatory
response.14
Another action of lethal toxin is to lyse macrophages, which are one of the body's important
defense mechanisms against invading organisms. Lethal factor is a zinc-binding protein with
metalloproteinase activity. The MAP kinase kinases Mek1 and Mek2 are macrophage proteins that
interact with it. Lethal factor cleaves Mek1 and Mek2 and an additional related factor MKK3. 15
Pretreatment of cultured peritoneal macrophages with inhibitors of intracellular calcium release
protects against anthrax lethal toxin cytotoxicity. Calcium release from intracellular stores may
be an essential step for the propagation of lethal toxin-induced cell damage in macrophages,
suggesting a potential way to prevent the toxicity from anthrax lethal toxin.16
Two recent papers in Nature (vol. 414, 8 Nov 2001) are of interest. The first, by A.D. Pannifer, et
al. (pp. 229-233), is "Crystal structure of the anthrax lethal factor". It identifies LF as a highly
specific proteinase that cleaves MAP kinase kinases (MAPKK), and details its complex with the N
terminus of MAPKK2.
The other paper, by K.A. Bradley, et al. (pp. 225-229) is "Identification of the cellular receptor for
anthrax toxin". The cellular receptor to which PA binds, and cloning of this receptor, are
described.
Researchers have found that EF is an adenylate cyclase that is activated by calmodulin at resting
state calcium concentrations in infected cells.17 EF has been purified and studied to determine
which residues are required for binding to anthrax PA.18
Another area of great interest is the development of rapid diagnostic tests for anthrax. Because
it is so important to begin treatment early, it is important to know whether a person with general
symptoms has anthrax or a less serious disease for which totally different treatments are
indicated.
A polymerase chain reaction (PCR) amplification on a microarray of gel-immobilized
oligonucleotides has been used for detection of bacterial toxins, including the anthrax toxin
genes.19 Other authors have investigated the molecular characterization of Bacillus anthracis by
use of multiplex PCR, enterobacterial repetitive intergenic consensus-PCR (ERIC-PCR), and
random amplification of polymorphic DNA (RAPD).20 The rpoB gene also has been used as a
specific chromosomal marker for real-time PCR detection of Bacillus anthracis. Variable region 1
of the rpoB gene was sequenced from 36 Bacillus strains, including 16 Bacillus anthracis strains
and 20 other related bacilli. Four nucleotides specific for Bacillus anthracis were identified. The
assay was specific for 144 Bacillus anthracis strains from different geographical locations and did
not cross-react with 175 strains of other related bacilli, with the exception of one strain. 21 Such
molecular methods, which examine the basic nature of the organism, could potentially be
developed into rapid diagnostic methods, providing answers in minutes or hours instead of days.
For epidemiological work, as has been done with recent terrorist incidents, it is important to know
whether anthrax bacilli isolated from different sources are the same or different strains. One
method for differentiating strains of Bacillus anthracis used long-range repetitive element
polymorphism-PCR. The authors examined five genetically distinct groups of diverse
geographical origin. All strains produced fingerprints of seven to eight bands, referred to as
skeleton bands, while one to three diagnostic bands differentiated between Bacillus anthracis
strains. The fingerprints of Bacillus anthracis showed very little in common with those of related
species such as Bacillus cereus, Bacillus thuringiensis, and Bacillus mycoides.22
Immunoassays can also be useful in detection of Bacillus anthracis. An immunoaffinity-based
phosphorescent sensor platform for the detection of bacterial spores has been developed. There
is interest in the production of field portable sensors for use by non-specialists. The
immunoaffinity column can capture spores. This is followed by the washing, elution and
phosphorescent detection of the spores. Spores are generically detected via the extraction of
dipicolinic acid followed by its chelation with terbium to yield a phosphorescent complex. 23
Vaccine
There is a human vaccine for anthrax, but it has been recommended only for people whose
occupation puts them at risk of encountering anthrax. In recent years, because of the threat of
bioterrorism, anthrax vaccine has been given to U.S. military personnel. It is not currently
recommended for use by the general civilian population. There has been much controversy over
the safety and effectiveness of the current vaccine.
Many people who receive the current vaccine may experience a mild flu-like illness and soreness
at the injection site, but systemic reactions are rare. A recent study among military personnel
estimated that 30% of recipients experience mild local reactions. One recipient experienced a
delayed and potentially serious life-threatening adverse reaction.24 There is great interest in
developing new anthrax vaccines that would contain only the antigen(s) needed for protection,
and not the portions of the cell that may cause reactions to the vaccine.
The Advisory Committee on Immunization Practices has issued recommendations concerning the
use of aluminum hydroxide adsorbed cell-free anthrax vaccine (Anthrax Vaccine Adsorbed) in the
United States.25 This vaccine was studied in a rabbit model. At 6 and 10 weeks, the quantitative
anti-protective antigen immunoglobulin G ELISA and the toxin-neutralizing antibody assays were
used to measure antibody levels to protective antigen. Rabbits were challenged at 10 weeks with
a lethal dose of anthrax spores by inhalation. All the rabbits that received the undiluted and 1:4
dilution of vaccine survived. Antibody levels to protective antigen at both 6 and 10 weeks were
significant predictors of survival.26 Passive transfer of lymphocytes and sera from mice
immunized using two different formulations of PA has been used to study the mechanism of
protection against Bacillus anthracis infection. These results also showed that an antibody
response may be important in protection against anthrax.27
In another study, guinea-pigs were immunized with PA and then challenged with a lethal dose of
anthrax spores. A direct correlation between survival and neutralizing antibody titer was found.
Passive transfer of hyperimmune sera showed the same relationship between neutralizing
antibody titers and protection. Such consistency was not found for antibody titers measured by
ELISA.28
Although most studies have concentrated on purification of PA for use as a vaccine, a study in
mice immunized with a plasmid encoding the lethal factor protein provided protection against a
challenge with anthrax lethal toxin.29
For the average citizen today, protection for anyone exposed to anthrax is through treatment
with doxycycline, ciprofloxacin, or penicillin. Vaccine is still in limited supply and is available for
those whose occupations may bring them in contact with anthrax, including military personnel in
locations where they are likely to encounter it.
© Copyright 2001, All Rights Reserved, CSA
1.
Bergey's Manual of Systematic Bacteriology, vol. 2, p. 1105, 1986, Sneath, P.H.A.; Mair, N.S.;
Sharpe, M.E.; Holt, J.G. (eds.); Williams & Wilkins, Baltimore, Maryland, USA
2.
"The capsule of Bacillus anthracis, a review." Ezzell, J.W., Jr.; Welkos, S.L.; J. Appl. Microbiol. ,
vol. 87, no. 2, p. 250, Aug 1999
3.
Microbiology. An Introduction. Fourth Edition, 1992, p. 566, Torto ra, G.J.; Funke, B.R.; Case,
C.L. Benjamin/Cummings Publishing Co., Inc., Redwood City, California, USA
4.
"Clinical aspects, diagnosis and treatment of anthrax." Friedlander, A.M., J. Appl. Microbiol. ,
vol. 87, no. 2, p. 303, Aug 1999
5.
"Anthrax." Dixon, T.C.; Meselson, M.; Guillemin, J.; Hanna, P.C.; N. Engl. J. Med. , vol. 141, no.
11, pp. 815-826, 9 Sep. 1999
back to article
6.
"Human ingestion of Bacillus anthracis-contaminated meat Minnesota, August 2000", Centers
for Disease Control and Prevention, Morb. Mortal. Weekly Rep. , vol. 49, no. 36, pp. 813-816,
Sep 2000
7.
"Clinical aspects, diagnosis and treatment of anthrax." Friedlander, A.M., J. Appl. Microbiol. ,
vol. 87, no. 2, p. 303, Aug 1999
8.
"Dehydroepiandrosterone and melatonin prevent Bacillus anthracis lethal toxin-induced TNF
production in macrophages", Shin, S.; Hur, G.-H.; Kim, Y.-B.; Yeon, G.-B.; Park, K.-J.; Park,
Y.-M.; Lee, W.-S.; Cell Biol. Toxicol. , vol. 16, no. 3, pp. 165-174, 2000
9.
"Anthrax toxins." Suesbery, N.S.; Wouds, G.F.V.; Cell. Mol. Life. Sci. , vol. 55, no. 12, pp.
1599-1609, Sep. 1999
10. "Trp 346 and Leu 352 residues in protective antigen are required for the expression of anthrax
lethal toxin activity", Batra, S.; Gupta, P.; Chauhan, V.; Singh, A.; Bhatnagar, R., Biochem.
Biophys. Res. Commun., vol. 281, no. 1, pp. 186-192, 16 Feb 2001
back to article
11. "A dominant negative mutant of Bacillus anthracis protective antigen inhibits anthrax toxin
action in vivo, Singh, Y.; Khanna, H.; Chapra, A.P.; Mehra, V.; J. Biol. Chem. , vol. 276, no. 25,
pp. 22090-22094, 22 June 2001
12. "Point mutations in anthrax protective antigen that block translocation", Sellman, B.R.; Nassi,
S.; Collier, R.J., J. Biol. Chem., vol. 276, no. 11, pp. 8371-8376, 16 Mar 2001
13. "Dominant-negative mutants of a toxin subunit: An approach to therapy of anthrax", Sellman,
B.R.; Mourez, M.; Collier, R.J., Science (Wash.), vol. 292, no. 5517, pp. 695-697, 27 Apr 2001
14. "Macrophage-derived cell lines do not express proinflammatory cytokines after exposure to
Bacillus anthracis lethal toxin," Erwin, J.L.; DaSilva, L.M.; Bavari, S.; Little, S.F.; Friedlander,
A.M.; Chanh, T.C.; Infect. Immun. vol, 69, no. 2, pp. 1175-1177, Feb 2001
15. "Lethal factor of Bacillus anthracis cleaves the N=terminus of MAPKKs: Analysis of the
intracellular consequences in macrophages," Pellizzari, R.; Guidi-Rontani, C., Vitale, G.; Mock,
M.; Montecucco, C., Int. J. Med. Microbiol., vol. 290, no. 4/5, pp. 421-427, 1 Dec 2000
back to article
16. "Intracellular calcium antagonist protects cultured peritoneal macrophages against anthrax
lethal toxin-induced cytotoxicity," Shin, S.; Hur, G.-H.; Kim, Y.-B.; Park, K.-J.; Park, Y.-M.; Lee,
W.-S.; Cell Biol. Toxicol., vol. 16, no. 2, pp. 137-144, 2000
17. "An extended conformation of calmodulin induces interactions between the structural domains
of adenylyl cyclase from Bacillus anthracis to promote catalysis," Drum, C.L.; Yan, S.Z.; Sarac,
R.; Mabuchi, Y.; Beckingham, K.; Bohm, A.; Grabarek, Z.; Tang, W.J.; J. Biol. Chem. , vol. 275,
no. 46, pp. 36334-36340, 17 Nov 2000
18. "Purification of anthrax edema factor from Escherichia coli and identification of residues
required for binding to anthrax protective antigen", Kumar, P.; Ahuja, N.; Bhatnagar, R.;
Infect. Immun. , vol. 69, no. 10, pp. 6532-6536, Oct. 2001
19. "PCR amplification on a microarray of gel-immobilized oligonucleotides: Detection of bacterial
toxin- and drug-resistant genes and their mutations," Strizhkov, B.N.; Drobyshev, A.L.;
Mikhailovich, V.M.; Mirzabekov, A.D.; Biotechniques, vol. 29, no. 4, pp. 844-857, Oct 2000
20. "Molecular characterizaation of Bacillus anthracis using multiplex PCR, ERIC-PCR and RAPD",
Shangkuan, Y.; Chang, Y., Yang, J.; Lin, H.; Shaio, M.; Lett. Appl. Microbiol. , vol. 32, no. 3, pp.
139-145, Mar 2001
back to article
21. "Utilization of the rpoB gene as a specific chromosomal marker for real-time PCR detection of
Bacillus anthracis", Oi, Y.; Patra, G.; Liang, X.; Williams, L.E.; Rose, S.; Redkar, R.J.;
DelVecchio, V.G.; Appl. Environ. Microbiol., vol. 67, no. 8, pp. 3720-3727, Aug 2001
22. "Use of long-range repetitive element polymorphism-PCR to differentiate Bacillus anthracis
strains, Brumlik, M.J.; Szymajda, U.; Zakowska, D.; Liang, X.; Redkar, R.J.; Patra, G.;
DelVecchio, V.G.; Appl. Environ. Microbiol. , vol. 67, no. 7, pp. 3021-3028, Jul 2001
23. "Immunoaffinity based phosphorescent sensor platform for the detection of bacterial spores",
Scholl, P.F.; Bargeron, C.B.; Phillips, T.F.; Wong, T.; Abubaker, S.; Groopman, J.D.; Strickland,
P.T; Benson, R.C.; Proc. Spie Int. Soc. Opt. Eng., vol. 3913, pp. 204-214, 2000
24. "Delayed life-threatening reaction to anthrax vaccine," Swanson-Biearman, B.; Krenzelok,
E.P.; J. Toxicol.: Clin. Toxicol.; vol. 39, no. 1, pp. 81-84, 2001
25. "Use of anthrax vaccine in the United States: Recommendations of the Advisory Committee on
Immunization Practices, Advisory Committee on Immunization Practices", J. Toxicol.: Clin.
Toxicol.; vol. 39, no. 1, pp. 85-100, 2001
back to article
26. "In vitro correlates of immunity in a rabbit model of inhalational anthrax", Pitt, M.L.M.; Little,
S.F.; Ivins, B.E.; Fellows, P.; Barth, J.; Hewetson, J.; Gibbs, P.; Dertzbaugh, M.; Friedlander,
A.M.; Vaccine, vol. 19, no. 32, pp. 4768-4773, 14 Sep 2001
27. "Passive transfer of protection against Bacillus anthracis infection in a murine model",
Beedham, R.J.; Turnbull, P.C.B.; Williamson, E.D.; Vaccine, vol. 19, no. 31, pp. 4409-4416, 14
Aug 2001
28. "Search for correlates of protective immunity conferred by anthrax vaccine", Reuveny, S.;
White, M.D.; Adar, Y.Y.; Kafri, Y.; Altboum, Z.; Gozes, Y.; Kobiler, D.; Shafferman, A.; Velan, B.;
Infect. Immun., vol. 69, no. 5, 2888-2893, May 2001
29. "Protection against anthrax lethal toxin challenge by genetic immunization with a plasmid
encoding the lethal factor protein", Price, B.M.; Liner, A.L.; Park, S.; Leppla, S.H.; Mateczun,
A.; Galloway, D.R.; Infect. Immun., vol. 69, no. 7, Jul 2001
Bacillus anthracis
Synonyms:
anthrax bacillus
Classification:
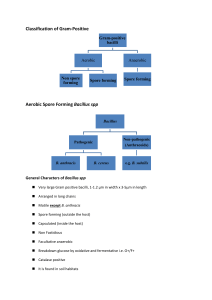
aerobic, gram+ bacteria, rods, in chains, sporulating
Diseases
Cutaneous anthrax
painless papule
ulcer
black eschar
edema
lymphadenopathy
bacteremia
fever
hypotension
death
Inhalation anthrax (Wool sorter's disease)
malaise
muscle aches
fever
dyspnea
hypoxia
meningitis
hypotension
respiratory failure
edema
death
mediastinal widening
Gastrointestinal anthrax
nausea
diarrhea
bloody diarrhea
fever
abdominal pain
hemorrhage
mesenteric adenitis
ascites
hypotension
death
Sites and Sources
animals, source
livestock, source
animal products, source bone meal, source
contaminated food, source
wool, source
soil, source
terrorists, source
bloodstream, pathogen
respiratory tract, pathogen GI tract, pathogen
skin, pathogen
multiorgan, pathogen
Diagnostic Factors
blood smear
aerobic growth
bamboo rod morphology
elliptic, central spores Medusa head colonies
no hemolysis on blood agar non-motile
gram positive rods in chains
PCR
Virulence Factors
spores
capsule
D-glutamic acid capsule
toxin
protective antigen
edema factor
lethal factor
Treatment and Prevention
penicillin
doxycycline
amoxicillin
vaccine
ciprofloxacin
Commentary
Anthrax is a zoonotic disease that can be acquired either by ingestion, inhalation, or skin
contact with contaminated animal products. Because the organism is a spore-former, hides,
hair, wool, and bone meal can all act as sources, as can fresh contaminated material. The
incubation period is usually from 1-6 days, but can be as much as 60 days. 8,000 to 50,000
spores must be inhaled for the inhalation form of the disease which is almost always lethal.
Cutaneous anthrax and gastrointestinal anthrax have lower fatality rates, but still must be
treated agressively to assure survival. Because of the stability of the spore in the
environment anthrax is one of the diseases commonly mentioned in relation to germ
warfare and terrorist activity. In 2001 several postal workers died of inhalation anthrax
after handling B. anthracis-laced mail. B. anthracis produces a powerful toxin of the A-B
type. It is composed of three factors; protective antigen (the B component), and two A
components, edema factor, and lethal factor. Edema factor is an adenylate cyclase
dependent on protective antigen for entry into the cell where it increases the concentration
of cAMP and disrupts normal cell function. The mechanism of action of lethal factor is
unknown. The unusual poly D-glutamic acid capsule is antiphagocytic, allowing large
numbers of bacilli to grow in the bloodstream.
Updated: October 28, 2003
http://medinfo.ufl.edu/year2/mmid/bms5300/bugs/bacanth.html 出處
MMID 網站
Bacillus anthracis (Anthrax)
Key Characteristics
Gram-positive rods
Non-hemolytic
Non-motile
Catalase positive
Spores present when cultured aerobically without CO2
ISOLATES WITH THE ABOVE CHARACTERISTICS SHOULD BE REPORTED TO THE PATIENT'S PH
SPECIES, UNABLE TO RULE-OUT B. ANTHRACIS. CONTACT YOUR STATE PUBLIC HEALTH LABO
INSTRUCTIONS ABOUT REFERRING THE ISOLATE OR SPECIMEN.
Colony Characteristics
Rapidly-growing colonies 2-5 mm overnight at 35°C
Non-pigmented, dry ground glass surface, flat or slightly convex, irregular edges with comma projections (Me
Sticky (tenacious) consistency. When teased with loop, will stand up like beaten egg white
Colonies are not hemolytic on sheep blood agar
Microscopic Characteristics
Large Gram-positive bacillus, singly and in chains. May become Gram-variable after 72 hours. May be encapsu
cultures
Terminal/subterminal spores do not swell vegetative cells. Spores may be seen on Gram stain, malachite gree
microscopy
Spores are not present in clinical material unless exposed to low CO2 levels, such as those found in the atmos
sporulation.
Safety
Biosafety Level 2 for processing clinical specimens
Biosafety Level 3 practices for all culture manipulations that might produce aerosols
Contact
If you suspect the above agent, immediately contact (in the order presented):
Drs. Lucy DesJardin and Mike Pentella at the University Hygienic Laboratory. If they aren't readily available, as
Virology, Serology, or Molecular laboratory. 319.335.4500 or 1.800.421.IOWA(4692)
University Hygienic Laboratory Duty Officer 319.335.4500 or 319.530.5981
As a final resort, the following 24-hour emergency hotlines have been established:
http://www.uhl.uiowa.edu/services/ter/biological/agents/anthracis.html 出處