Unit 1 Due
advertisement
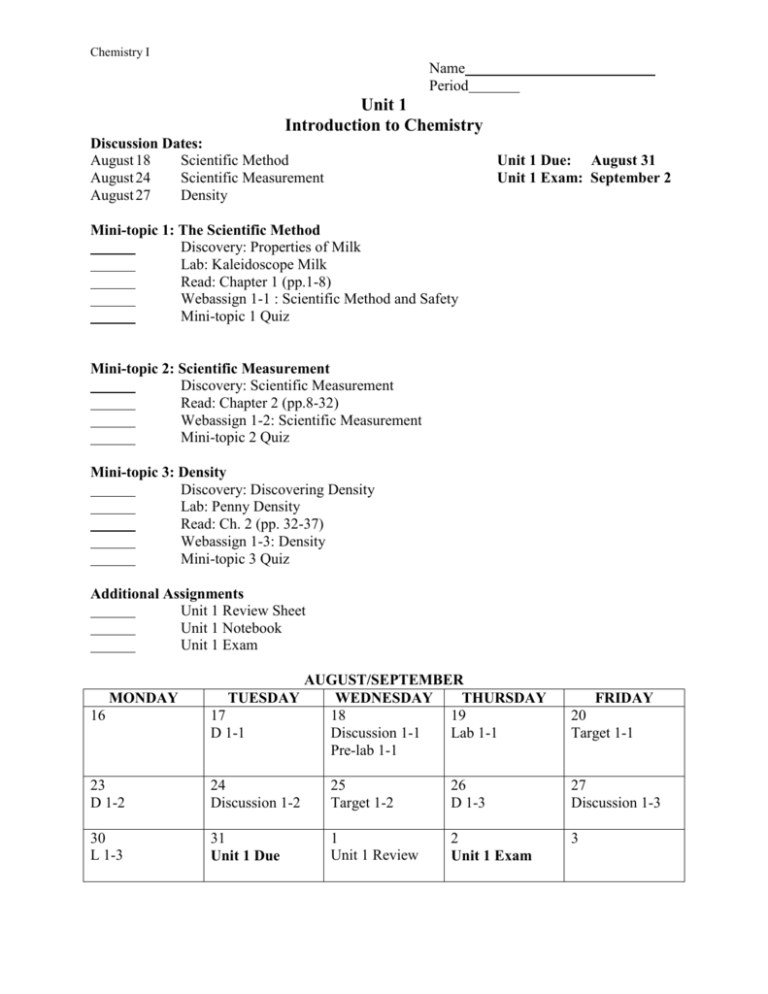
Chemistry I Name Period Unit 1 Introduction to Chemistry Discussion Dates: August 18 Scientific Method August 24 Scientific Measurement August 27 Density Unit 1 Due: August 31 Unit 1 Exam: September 2 Mini-topic 1: The Scientific Method Discovery: Properties of Milk Lab: Kaleidoscope Milk Read: Chapter 1 (pp.1-8) Webassign 1-1 : Scientific Method and Safety Mini-topic 1 Quiz Mini-topic 2: Scientific Measurement Discovery: Scientific Measurement Read: Chapter 2 (pp.8-32) Webassign 1-2: Scientific Measurement Mini-topic 2 Quiz Mini-topic 3: Density Discovery: Discovering Density Lab: Penny Density Read: Ch. 2 (pp. 32-37) Webassign 1-3: Density Mini-topic 3 Quiz Additional Assignments Unit 1 Review Sheet Unit 1 Notebook Unit 1 Exam 16 AUGUST/SEPTEMBER TUESDAY WEDNESDAY THURSDAY 17 18 19 D 1-1 Discussion 1-1 Lab 1-1 Pre-lab 1-1 FRIDAY 20 Target 1-1 23 D 1-2 24 Discussion 1-2 25 Target 1-2 26 D 1-3 27 Discussion 1-3 30 L 1-3 31 Unit 1 Due 1 Unit 1 Review 2 Unit 1 Exam 3 MONDAY Chemistry I Unit 1 Objectives 1-1 The Scientific Method 1. Define chemistry. 2. List and describe the steps in the scientific method. 3. Distinguish between a theory and a scientific law. 4. Observe appropriate safety precautions when doing laboratory investigations. Manipulate laboratory equipment properly. 1-2 Scientific Measurement 5. Distinguish between quantitative and qualitative measurements. 6. Convert measurements into scientific notation. 7. Distinguish between the accuracy and precision of a measurement. 8. State the fundamental units of measurement. 9. Calculate the percent error of a measurement. 1-3 Density 10. Understand the physical property of density. 11. Calculate the density of an object from experimental data. 12. Distinguish between intensive and extensive properties. Explain why density is an intensive property. Unit 1 Discussion Questions These questions are based on the discovery activities and reading assigned in the textbook. Students are expected to actively participate in the class discussions by answering questions, asking questions, and listening to and responding to other students’ ideas. 1-1 The Scientific Method 1. What is chemistry and why is it considered to be the “central science?” 2. What do chemists study? 3. How is chemistry related to your life? 4. What is meant by the scientific method? 5. What is the difference between a theory and a scientific law? 1-2 Scientific Measurement 1. Explain the difference between qualitative and quantitative observations. 2. How can we make quality scientific measurements? 3. What is meant by the “uncertainty of a measuring device?” 4. How is the uncertainty of a measuring device related to the precision of your measurements? 5. What can percent error tell you about the accuracy of your measurements? 1-3 Density 1. What is density? 2. Will it sink or will it float? 3. How do you measure density? 4. How do you calculate density from measured data? 5. Is density an intensive or extensive property? Provide experimental justification.










