Properties of Matter-2
advertisement
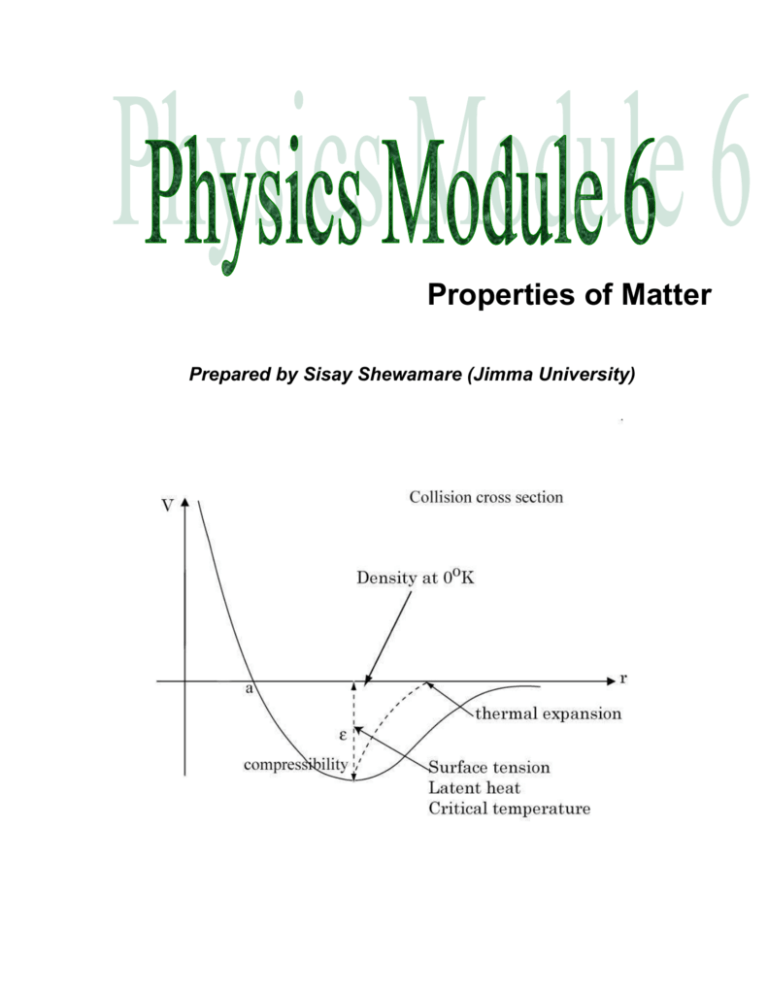
Properties of Matter Prepared by Sisay Shewamare (Jimma University) NOTICE TABLE OF CONTENTS FOREWORD This module has four major sections The first one is the INTRODUCTORY section that consists of five parts vis: 1. TITLE:- 2. PRE-REQUISITE KNOWLEDGE: In this section you are provided with infromation regarding the specific pre-requisite knowledge and skills you require to start the module. Carefully look into the requirements as this will help you to decide whether you require some revision work or not. 3. TIME REQUIRED: It gives you the total time (in hours) you require to complete the module. All self tests, activities and evaluations are to be finished in this specified time. 4. MATERIALS REQUIRED: Here you will find the list of materials you require to complete the module. Some of the materials are parts of the course package you will receive in a CD-Rom or access through the internet. Materials recommended to conduct some experiments may be obtained from your host institution (Partner institution of the AVU) or you may acquire, borrow or by some other means. 5. MODULE RATIONALE: In this section you will get the answer to questions like “Why should I study this module as pre-service teacher trainee? What is its relevance to my career?” The second is the CONTENT a section that consists of three parts: 6. OVERVIEW: The content of the module is briefly presented. In this section you will find a video file (Quicktime, .move) where the author of this module is interviewed about this module. The paragraph overview of the module is followed by an outline of the content including the approximate time requiered to complete each section. A graphic organization of the whole content is presented next to the outline. All these three will assist you to picture how content is organized in the module. 7. GENERAL OBJECTIVE(S): Clear, informative, concise and achievable objectives are provided to give you what knowledge skills and attitudes you are expected to attain after studying the module. 8. SPECIFIC LEARNING OBJECTIVES (INSTRUCTIONAL OBJECTIVES): Each of the specific objectives, stated in this section, are at the heart of a teaching learning activity. Units, elements and themes of the module are meant to achieve the specific objectives and any kind of assessment is based on the objectives intended to be achieved. You are urged to pay maximum attention to the specific objectives as they are vital to organize your effort in the study of the module. The third section is the bulk of the module. It is the section where you will spend more time and is refered to as the TEACHING LEARNING ACTIVITIES. The gist of the nine components is listed below: 9. PRE-ASSESSMENT: A set of questions, that will quantitatively evaluate your level of preparedness to the specific objectives of this module, are presented in this section. The preassessment questions help you to identify what you know and what you need to know, so that your level of concern will be raised and you can judge your level of mastery. Answer key is provided for the set of questions and some pedagogical comments are provided at the end. 10. KEY CONCEPTS: This section contains short, concise definitions of terms used in the module. It helps you with terms with which you might not be familiar to the module. 11. COMPULSORY READINGS: A minimum of three compulsory reading materials are provided. It is mandatory to read the documents. 12. COMPULSORY RESOURCES: A minimum of two video, audio with an abstract in text form is provided in this section. 13. USEFUL LINKS: A list of atleast ten websites is provided in this section. It will help you to deal with the content in greater depth.. 14. TEACHING AND LEARNING ACTIVITIES: This is the heart of of the module. You need to follow the learning guidance in this section. Various types of activities are provided. Go through each activity. At times you my not necessarily follow the order in which the activities are presented. It is very important to note: formative and summative evaluations are carried out thoroughly all compulsory readings and resources are done as many as possible useful links are visited feedback is given to tha author and communication is done Enjoy your work on this module. I. Properties of Matter BY SISAY SHEWAMARE JIMMA UNIVERSITY ETHIOPIA II PRE - REQUISITE COURSE OR KNOWLEDGE In order to study this module, you need to complete the modules on Mechanics I, Mechanics II, and Electricity and Magnetism. This module also assumes you have taken introductory course in Calculus. III TIME The time you require to complete this module is 120hrs. For chapterwise breakdown see section 6 of the module IV MATERIALS INTERNET CONNECTION COMPULSORY READINGS AND COMPULSORY RESOURCES (As listed in sections 11 & 12) STANDARD WEIGHTS WIRES MADE OF DIFFERENT SUBSTANCES SOFTWARE PACKAGE V MODULE RATIONALE Science teaching in secondary schools is expected to enable learners to work in scientific ways (apply scientific principles), stimulate their curiosity and deepen their interest in the natural and physical world. In this module you will study the behavior of solids when subjected to strains, and the behavior of fluids in different contexts is studied. You will also understand the thermal and electrical conductivity (also known as transport properties) of metals. The study of mechanical, thermal and electronic properties of materials will not only help you for advanced studies in solid state Physics and electronics physics, but also will give you a cutting edge in teaching technological applications of the Physical Sciences for your future students. * Fig: Which properties of Tungsten wire make it very convenient for the construction of a bulb fillament? VI OVERVIEW In this module you will study elastic and transport properties of materials like elasticity, fluid flow, diffusion, osmosis, thermal and electrical conductivities of a materials At the beginning, activities leading you through the details of the effects of force on various types of materials are presented. Then you will come across activities that will enable you describe the properties of fluids and use these properties to arrive at principles and laws such as Archimedes principle, Pascals law and Bernoull’is equation. The module includes properties like viscosity, diffusion, thermal properties conductivity, expansion), Electrical conductivity of metals, semiconductors and alloys. These properties are also known as transport properties. Image shutterstock_47271 6.1 Out Line 1. Elasticity Load and strees; strain Stress Strain relationship:Hooke’s law Compressibility, Elasticity and Plasticity Young’s modulus Poisson’s ratio 2. Fluids Density Pressure Fluid at rest Measuring pressue Pascal’s Principle Archimedes Principle Equilibrium of floating object Bernoulli’s equation The flow of real fluid 3. Transport properties Diffusion ( 30hours) (45 hours) (45 hours) 6.2 Viscosity Thermal conductivity Thermal expansion Electrical conductivity of metals, semiconductors and alloys. Graphic Organizer A. Elasticity Viscosity Diffusion conductivity Expansion Thermal Properties Metals Semiconductors C. Transport Properties Properties of Matter Electrical conductivity Alloys B. Fluids Stress. Strain Compressibility Plasticity Young's Modulus Poson Ratio Density Pressue Fluids at rest Measuring Pressue Pascal's Principle Archimedes Principle Equilibriumof floating objects Equation ofContinuity Bernoulli's Equation The flow of real fluids VII. General Objective(s) After completing this module you would be able to: Explain the concept of elastic properties of materials Describe the transport properties of materials Appreciate the properties of fluids and apply the concepts to a range of contexts. Use thermal conductivity of matteials to solve porblems Use Elcectrical conductivity of materials to solve problems. VIII. Specific Learning Objectives (Instructional Objectives) content Learning objectives After Completing this section you would be able to: 1 Elasticity (35 hours) Determine the effect of force on materials Load and strees; strain Calculate Young’s modulus for a range of materials Stress Strain relationship:Hooke’s law Calculate Poisson’s ratio for a Compressibility, Elasticity and given material Plasticity Predict material properties Young’s modulus Poisson’s ratio 2 Fluids (45 hours) Density Pressure Fluids at rest Measuring pressue Pascals Principle Archimedes Principle Equilibrium of floating object Bernoulli’s equation The flow of real fluids 3 Transport properties (45 hours) Diffusion Viscosity Thermal conductivity Thermal expansion Electrical conductivity of metals, semiconductors and alloys. Describe basic properties of fluid (density,pressure) Apply the properties of fluids (Archimedes principle, Pascal’s law) Evaluate fluid motion (continuity,turbulance real fluids ) Use Bernoulli’s equation Analyse particle motion in fluids Describe relative properties of solids, liquids and gases Evaluate the effects of heat on materials e.g. calculate thermal expansion Calculate the effective concentration of mobile electrons in metals, alloys and semiconductors IX. PRE-ASSESSMENT This pre assessment questions comprizes questions from the prerequisite knowledge as well as question that assess your mastery level of the objectives stated in this module. If your performance is more than 70% you can proceed to work on this module. However if your performance is less than 70% you need to revise some of your school Physics. The depth of the revision work you need is proportional to how far your performance is away from the required minimum Answers to the questions are provided immediately after the questions. * How does air support an aircraft?. 9.1 QUESTIONS 1. Figure 1 the weight of the liquid, density , at x is kept constant while the liquid flows out of the narrow tube at depth h below x. The velocity v of the liquid from the narrow tube is a) ; h g b) 2gh c) x h 2gh v d) gh e) . 2gh 2. A hot air balloon moving upwards has a total weight of 200N and a volume of 20m3. Assuming the air density of 1.2kgm-3, the net upward force on the balloon in N is then about a) .24 b) 36 c) 40 d) 176 e) 240 3. When a stone of mass m at the end of a string is whirled in vertical circle at constant speed a) The tension (force) in the string stays constant b) The tension is least when the stone reaches the bottom of the circle c) The tension in the string is always mg d) the weight mg is always the centripetal force e) the tension is greatest when the stone is at the bottom of the circle 4. At the olympic high-diving competition, a diver from the top board curves her body in order to a) dive cleanly in to the water d) spin more slowly b) spin more e) increase her speed c) increase her energy 5. When streached beyound its elastic limit, a metal rod such as steel a) becomes plastic c) obeys Hooke’s b) has no energy d) becomes colder 6. Figure 2 shows three mass in a row. The force on the 1kg mass is zero if the distance x in meters is a) 2 1kg b) 3 c) 4 x 4kg 9kg 15 d) 5 e) 6 Figure 2 7. The time constant of the circuit shown in Figure 3 is 4s. The time constant of the circuit shown in figure 4 is thus: a) 8s b) 4s c) 2s R C Figure 3 C C R Figure 4 R d) 1s e) 0.5s 8. At what temperature are the reading from a Fahrenheit thermometer and Celisius thermometer the same. a) -20 c) 32 b) 40 d) -40 e) 72 9. Which of the following are semiconductor materials? a) gallium arsenide c) silicon b) germanium d) all of the A above 10. Why are semiconductors valuable in modern electronics ? a) use low power c) fast switching b) reliable d) all of the above 11. Which electronic devices are primarily made from semiconductors ? a) transistors c) capacitors b) .resistors d) none of the above 12. How does the conductivity in pure semiconductors vary with temperature? a) conductivity increases as temperature goes down b) conductivity increases as temperature goes up c) conductivity does not change with temperature 13. What explains why semiconductors have different electrical properties from metals? a) more valence electrons c) band gap structure b) fewer valence electrons d) no differences 14. Both _electrons _ and _holes_ are considered charge carriers. 15. A diode contains both _n-type_ and __p-type_ regions. 9.2 1) 2) 3) 4) 5) 9.3 ANSWER KEY C C E B A 6) 7) 8) 9) 10) E B D D D 11) 12) 13) 14) 15) A B C electron hole n-type p-type PEDAGOGICAL COMMENT FOR LEARNERS The module is presented in such a way that you will find yourself in a variety of activities like reading, going through worked examples, experimenting virtually and in the real lab, online discussion with study group, solving problems etc. This is possible partly by the package you receive with this module and via the internet. Your effort to experience all compulsory materials and as many resources as possible has no substitute. Infact learning takes place with the learner’s effort. Therefore you are advised to work all the problems provided and consult the references suggested. The concepts presented are best understood in experimental tests. It is a very good idea if you keep in touch with the AVU partner University. The last thing you have to do is evaluate yourself whether you have achieved the expected learning outcomes mentioned at the begining of the module. X KEY CONCEPTS (GLOSSARY) 1. ELASTICIY: Is the property of a material, or a substance, or a body of returning to its original size and shape after distortion or deformation by a force. (Source Wikipedia consulted on … ) 2. STRESS: Is a force per unit area, measured in newtons per meter squared ( Nm -2 ). Examples of a stress include a tension, a thrust, and a shearing force. 3. STRAIN: Is the ratio of the dimensional change produced to the original dimension. When a stress is applied to a body a strain is produced. The body can be distoreted or deformed, depending upon its elesticy. It may be a ratio of lengths, areas, or volumes. 4. YOUNG’S MODULUS: Is the modulus of elastticty of a wire or rod stretched longitudinally, or of a rod compressed longitudinally. It is measured in N m 2 Force F Stress Area A Extension x Strain= Length l Stress Fl Youngs Modulus E Strain Ax 5. COMPRESSIBILITY: In thermodynamics and fluid mechanics, compressibility is a measure of the relative volume change of fluid or solid as a response to a pressure (or mean stress) change. 1 V V P where V is volume and P is pressure. The above statement is incomplete, because for any object or system the magnitude of the compressibility depends strongly on whether the process is adiabatic or isothermal. 6. PLASTICITY: Is the property of a material, or a substance, of being permanently deformed by a force, without breaking. 7. POISSON RATIO: When a sample of material is stretched in one direction, it tends to get thinner in the other two directions. Poisson's ratio ( , µ), named after Simeon Poisson, is a measure of this tendency. Poisson's ratio is the ratio of the relative contraction strain, or transverse strain (normal to the applied load), divided by the relative extension strain (in the direction of the applied load). For a perfectly incompressible material, the Poisson's ratio would be exactly 0.5. Most practical engineering materials have ? between 0.0 and 0.5. Cork is close to 0.0, most steels are around 0.3, and rubber is almost 0.5. Some materials, mostly polymer foams, have a negative Poisson's ratio; if these auxetic materials are stretched in one direction, they become thicker in perpendicular directions. Assuming that material is compressed along y axis v yx x y v where yx is the resulting Poisson's ratio, x is transverse strain, and y is axial strain. 8. PASCAL’s PRINCIPLE: A change pressure applied to an enclosed fluid is transmitted undiminished to every point of the fluid and the walls of the containing vessel. 9. ARCHIMEDE’S PRINICIPLE: Any body completely or partially submerged in a fluid is buoyed up by a force equal to the weight of the fluid displaced by the body 10. BERNOULLI’S EQUATION: As a fluid moves through a pipe of varying cross section and elevation, the pressure will change along the pipe. 11. VISCOSITY: Is resistance to the internal friction between molecules. Viscosity can be measured by an instrument called a viscometer. One way to measure relative viscosity of liquids is to use a 5 ml pipette and a stop watch. Draw up precisely 5.00 ml of the liquid and begin the stop watch as the liquid leaves the pipette. The longer it takes to empty the more viscous is the liquid. Some liquids like water have a low viscosity where other liquids like honey have a high viscosity. Viscosity will be affected by the temperature. At higher temperatures the viscosity decreases as the molecules take on more kinetic energy allowing them to move past each other faster 12. DIFFUSION: Diffusion is the movement of particles from higher chemical potential to lower chemical potential (chemical potential can in most cases of diffusion be represented by a change in concentration). An electric charge is an attribute of matter that produces a force 13. THERMAL CONDUCTIVITY: Thermal expansion of solids:or a body is a consequence of the change in the average separation between its constituent atoms or molecules. 14. ELECTRICAL CONDUCTIVITY: Is a measure of a material's ability to conduct an electric current when an electrical potential difference is appplied across the conductor. Its movable charges flow, giving rise to an electric current. The conductivity σ is defined as the ratio of the current density to the electric field strength J E , XI COMPULSORY READINGS Reading #1 Mechanical Properties Complete reference : http://dmoz.org/Science/Physics/Fluid_Mechanics_and_Dynamics/ Abstract : The links on the above mentioned page lead you to html materials on topics of Bernoulli's Principle Animation, Calculations and Equations of Fluid Mechanics, Classical Fluid Mechanics Problem Solutions - Solutions to Classical Fluid Flow & Momentum Transfer Problems, Fluid dynamics course material, Fluid Mechanics, and many more that are directly relevant to this module. Rationale: The Open Directory Project is the largest, most comprehensive human-edited directory of the Web. It is constructed and maintained by a vast, global community of volunteer editors. Date consulted: October, 2006 Reading #2 Gases Liquids and Solids Complete reference http://en.wikipedia.org/wiki/Elasticity_%28physics%29 Abstract : The topics discussed in this document include Contents Modeling elasticity, Transitions to inelasticity Rationale: This is one chapter of a free text book maintained by www.lightandmatter.com It is available in pdf and html formats. The pdf files can be downloaded chapter by chapter d potential; introduction to special relativity; Maxwell's equations, in both differential and integral form; and properties of dielectrics and magnetic materials Date consulted: September, 2006 Reading #3 Solid Mechanics Complete reference :http://en.wikibooks.org/wiki/Solid_Mechanics#Stress Abstract : Topics in this reading material follows the continuum mechanics approach, where the material properties to be the same even when we consider infinitesimal areas and volumes. The alternative approach is to build up material properties from basic equations relating atomic forces and interactions, and extending it to larger sets of such entities (e.g., molecular dynamics).. Rationale: This is part of a book on solid mechanics and it is a good reading material for this module. Date consulted: Nov, 2006 XII COMPULSORY RESOURCES 1. Resource #1: Effect of Temprature and Volume on the number of Collisions Source;Lon-CAPA url:-: http://lectureonline.cl.msu.edu/~mmp/kap10/cd283.htm. Date Consulted:- Nov 2006 Description:- This Java applet helps you understand the effect of temperature and volume on the number of collisions of the gas molecules with the walls. In the applet, you can change the temperature and volume with the sliders on the left side. You can also adjust the time for which the simulation runs. The applet counts all collisions and displays the result after the run. By varying temperature and volume and keeping track of the number of collisions, you can get a good feeling of what the main result of kinetic theory will be. 2. Resource #2 Virtual Experiment on the Ideal Gas Law Source;Uoregon University url:-: http://jersey.uoregon.edu/vlab/Piston/index.html Date Consulted:-Nov 2006 Description:- This Java applet helps you to do a series of virtual experiments, you will control the action of a piston in a pressure chamber which is filled with an ideal gas. The gas is defined by four states: Temperature; Volume or density; Pressure and Molecular Weight There are 3 possible experiments to do. In the third experiment, labelled Ideal Gas Law, you can select from the Red, Blue or Yellow gas containers. Each gas in those containers has a different molecular weight and hence each will respond differently under changing pressure conditions.. 3. Resource #3 Computer Calculation of Phase Diagrams Source: video.google.com Complete Reference: http://video.google.com/videoplay?docid=1397988176780135580&q=Thermodynamic s&hl=en Rationale: Thermodynamic models of solutions can be used together with data to calculate phase diagrams. These diagrams reveal, for a given set of all parameters (such as temperature, pressure, magnetic field), the phases which are thermodynamically stable and in equilibrium, their volume fractions and their chemical compositions. This lecture covers the pragmatic methods implemented in commercial software for the estimation of multicomponent, multiphase equilibria. The content should be generally useful to scientists. This is the fifth of seven lectures on the thermodynamics of phase transformations XIII USEFUL LINKS Useful Link #1 Title: Buoyant Force in Liquids URL: http://www.walter-fendt.de/ph11e/buoyforce.htm Screen Capture: Description: This Java applet shows a simple experiment concerning the buoyancy in a liquid: A solid body hanging from a spring balance is dipped into a liquid (by dragging the mouse!). In this case the measured force, which is equal to the difference of weight and buoyant force, is reduced. You can change (within certain limits) the preselected values of base area, height and densities by using the appropriate text fields. Rationale: This virtual experiment conforms with activity 2 of the module. Useful Link #2 Title: Water Pressure and depth. URL: http://www.mste.uiuc.edu/murphy/PicnicCooler/default.html Screen Capture: Description: This applet was written by Lisa Denise Murphy at the University of Illinois. Early drafts were written in 1999. The current version was last revised in January of 2000. Permission is given for students and teachers to use this applet, provided acknowledgement is made of the source. Rationale: This virtual activity is of use for activity 2 Useful Link #3 Title: Solid Mechanics URL: http://en.wikibooks.org/wiki/Solid_Mechanics Screen Capture: Description: This is a book on solid mechanics. . Rationale: The contents of activity 1 and activity 3 are entertained in greater detail Useful Link #4 Title:Viscosity URL: http://www.spacegrant.hawaii.edu/class_acts/ViscosityTe.html Screen Capture: Description: This is advanced description of viscosity for more curious readers. Rationale: Useful Link #5 Title: Thermal Conductivity URL: http://hyperphysics.phy-astr.gsu.edu/hbase/thermo/thercond.html Screen Capture: Description: An excellent presentation with many relevant liniks. Rationale: supplements activity 2 XIV. TEACHING AND LEARNING ACTIVITIES ACTIVITY 1: Elasticity of Materials You will require 30 hours to complete this activity. In this activity you are guided with a series of readings, Multimedia clips, worked examples and self assessment questions and problems. You are strongly advised to go through the activities and consult all the compulsory materials and as many as possible among useful links and references. Specific Teaching and Learning Objectives Analyse the effects of force on materials Define different types of coefficient of elasticity Summary of the Learning Activity In this activity, you will define the concepts of load, strees and strain. You will also derive the mathematical equations for the stress and strain. In addition you will be able to solve different problems. The simplest cases of deformations are those i) in which a wire, fixed at its upper end, is pulled down by a weight at lower end, bringing about a change in its length. ii) in which an equal compression is applied in all directions, so that there is a change of volume but no change in shape. iii) in which a system of forces may be applied to a body such that, although there is no motion of the body as a whole, there is relative displacement of its contiguous layers, causing a change in shape or “form” of the body with no change in its volume. In all these cases the body is said to be Strained or deformed Key Concepts Load: The term load, in the present context, implies the combination of external forces (for example the weight of the body itself, together with those connected with it; centrifuge forces in the case of rotating wheels and pulleys; forces due to friction or forces due to unequal expansion and contraction on changes of temperature etc.) acting on a body and its effect is to change the form or the dimensions of the body. Stress: The restoring or recovering force per unit area set inside the body is called strees. Strain: The change produced in the dimensions of a body under a system of forces or couples in equilibrium, is called strain, and is measured by the change per unit length (linear strain), per unit volume, (volume strain), or the angular deformation, (shear strain, or simply shear) according as the change takes place in length, volume or shape of the body. Linear Elasticity: (also known as elasticity of length ) Is a property possessed by bodies that increase in length when a tensile force is applied to the. The applied force causes equal and an opposite force called restoring or recovering force set insite the body. Poisson Ratio: The Poisson's ratio is related to elastic moduli K, the bulk modulus; n as the shear modulus; and Y, Young's modulus, by the following. The elastic moduli are measures of stiffness. They are ratios of stress to strain. Stress is force per unit area, with the direction of both the force and the area specified.restoring or recovering force per unit area set inside the body is called strees. Compressibility: The Bulk Modulus is sometimes referred to as compressibility; so that, 1 compressibility of a body is equal to where k is its Bulk modulus. it must thus k be quite clear that whereas Bulk modulus is stress per unit strain, compressibility represents strain per unit stress restoring or recovering force per unit area set inside the body is called strees. List of Relevant Readings 1. Reference: Nelkon & Parker (1995), Advanced Level Physics, 7th ed, CBS Publishers & Ditributer, 11, Daryaganji New Delhi (110002) India. ISBN 81-2390400-2. Rationale: This reading assumes high school physics background of the reader it suits this module 2. Reference: Flower B.H., Mendoz E (1970), Properties of Matter. John Wiley & Son Ltd, ISBN 0471 26498 9R McCliment (1984). Phusics, Harcourt Brace Jovanovich, Publishers, San Diogo . Rationale: This reading provide easy sources of information. The contents have been treated in lucid manner with adequate mathematical support. 3. Reference: Grant Mathur D.S. (1985), Elements of Properties of Matter, Shaym Lal Charitable Trust, Ram Nagar, New Delhi 110055. 284-360 Rationale: List of Relevant Resources 1. Reference: http://hyperphysics.phy-astr.gsu.edu/hbase/permot3.html 2. Reference:- http://en.wikipedia.org/wiki/Young's_modulus Summary: Young's Modulus (E) (also known as the Young Modulus, modulus of elasticity, elastic modulus or tensile modulus) is a measure of the satiffness of a given material. It is defined as the ratio, for small strains, of the rate of change of stress with strain Rationale: Reference: http://en.wikipedia.org/wiki/Elasticity_of_substitution 3. Summary: An important property of many structural materials is their ability to regain their original shape after a load is removed. These materials are called elastic. Rationale:- List of Relevant Useful Links 1. Title: Elasticity URL: http://en.wikipedia.org/wiki/Young's_modulus Abstract:- properties and mathematical equation is found 2. Title: work done in strain URL: http://en.wikipedia.org/wiki/Young's_modulus Abstract: equation of work done Introduction to the Activity All bodies can, more or less, be deformed by suitably applied force. The simplest cases of deformation that you can do are the following 1. In which a wire, fixed at its upper end, is pulled down by a weight at lower end, bringing about a change in its length L A(cross-section) L F (Load attached) (a) Figure 1.1 System of forces and deformations defining elastic modulus of linear tension 2. In which an equal compression is applied in all directions, so that there is a change of volume but no change in shape. F V V F F F (b) Figure 1.2 System of forces and deformations defining elastic modulus of a change in volume 3. A system of forces may be applied to a body such that, although there is no motion of the body as a whole, there is relative displacement of its contiguous layers, causing a change in shape or “form” of the body with no change in its volume F B L B’ L A (c) C’ D Figure 1.3 System of forces and deformations defining elastic modulus due to tangential forces producing an angle of shear Detailed Description of the Activity (Main Theoretical Elements) *Insure clear learning guidance and variety of learning activities are provided throughout the acitvity. 1: Elasticity In all the above cases the body is said to be strained or deformed. When the deforming forces are removed the body tends to recover its original condition. For example, the wire, in the Figure 1.1, tends to come back to its original length when the force due to the suspended weight is removed from it, or, a compressed volume of air or gas throws back the piston when it recovers its original volume. This property of a material body to regain its original condition, on the removal of the deforming forces, is called elasticity. Bodies, which can recover, completely their original condition, on the removal of the deforming forces, are said to be perfectly elastic. On the other hand, bodies, which do not show any tendency to recover their original condition are said to be plastic. 1.1 Linear elasticity, Linear elasticity also known as elasticity of length, is a property possessed by bodies that increase in length or breadth or width when a tensile force is applied to them normally in those directions. Young’s Modulus When the deforming force is applied as shown in the Figure 1.1 to the body only along in particular direction, the change per unit length in that direction is called longitudinal, linear or elongation strain, and the force applied per unit area of cross–section is called L F F .L longitudinal or linear stress . Young's modulus Y = . For uniform change Y a a. L dF = . . For non uniform change Where a is the cross sectional area of the rod, L is the a d length of the rod, F is the Load . Stress: Is the tensile force per unit area and is denoted by σ. F Young’s modulus, E A e FL for a uniforn change. eA l L dF where A is the cross-sectional area of the rod, l A dl is the length of the rod and, F, is the load. For non uniform change E Bulk modulus. Here, the force is applied normally and uniformly as shown in the Figure1.2 to the whole surface of the body; so that, while there is a change of volume, there is no change of F shape The force applied per unit area, (or pressure), gives the Stress and the change A v per unit volume, the strain= their ratio giving the Bulk Modulus for the body. k V F F .V V a P v a.v v V Modulus of Rigidity. In this case, while there is a change in the shape of the body, there is no change in this volume as shown in the Figure 1.4 Tangential force F is applied in the direction shown point B shifts to B’, D to D’, i.e. the lines joining the two faces turn through an angle .the face ABCD is then said to be sheared through an angle this angle (in radians), through which a line originally perpendicular to the fixed face is turned, gives the strain or BB ' l the shear strain, or the angle of shear, as it is often called as can be seen, = = , AB L where l is the displacement BB’ and L, the length of the side AB or the height of the cube. In otherwords, =relative displacement of plane AB’D’C distance from the fixed plane ABCD. Tangential stress is equal to the force F divided by the area of the face BDdb( F area=a),i.e. equal to . The ratio of the tangintial stress to the shear strain gives the a F F F .L a a coefficient of rigidity of the material of the body denoted by n= If the a. L dF a shearing strain is not proportional to the shear stress applied, we have n= d b’ b B L A l B’ F D a C Figure1.4 Module regidity d D’ d’ c Work done in a strain In order to deform a body, work must be done by the applied force. The energy so spent is strored up in the body and is called the energy of strain. When the applied forces are removed the stress disappears and the energy of strain appears as heat. Let us consider the work done during the three cases of strain. Elongation strain-(stretch of a wire) Then work done W= F.dl Now, Young’s modulus for the material of the wire, i.e. E F .L a.l where L- is the original length l - the increase in length a- cross sectional area F- the force applied Then the force applied F E.a.l L The work done during the stretch from 0 up to l l w 0 E.a ldl L l E.a ldl = L 0 = E.a l 2 L 2 E.a.l 1 E.a.l = .l But F L 2 L Hence w 1 Fl 2 1 = (stretching force x stretch) 2 Work done per unit volume = = 1 l F. 2 L.a 1F l . 2 a L 1 = stress x strain 2 Volume Strain Let σ be the stress applied. Then, over an area a the force applied is σ.a, and therefore, work done for a small movement dx, in the direction of σ, is equal to σ.a.dx. Now, a.dx is equal to dv, the small change produced in volume. Thus, work done for a change dv is equal to σ dv. And, therefore total work done for the whole change in volume, from 0 to V, is given by V W dV 0 K . V V ; so that K . v v Where V is the original volume and K is the Bulk modulus v k.v dv V 0 and w v k VdV V 0 = = 1 k .v .v 2 V = 1 v 2 = 1 stress x change in volume 2 1 v Work done per unit volume = 2 V = 1 stress x strain 2 Shearing Strain Consider a cube of edge L,(Fig.(1)), with its lower foce DC fixed, and let F be the tangential force applied to its upper face in the plane of AB, so that the face ABCD is distorted into the position A’B’CD or sheared through an angle . Let the displacement AA’ be equal to BB’= l . Then, work done during a small displacement d l is equal to F.d l . And, therefore work done for the whole of the displacement, from 0 to l is given by l w F .dl 0 Now n F , F n.a. and a L2 , a. also l L where L is the length of each edge of the cube so that F n.L2 . l = n.L.l L Work done during the whole stretch from 0 to l , i.e l w n.L.l.dl 0 = 1 1 1 n.L.l 2 = F.l = tangential force x displacement 2 2 2 Work done per unit volume = = 1 F .l 1 F l 1 F . = = 2 L3 2 L2 L 2 a 1 stress x strain. 2 Thus, we see that in any kind of strain, work done per unit volume is equal to strain Dimensions. The strain of a wire has no dimenssion The dimenssion of stress= ML1T 2 The SI unit of modulus of elasticity is the Pascal 1 stress x 2 Task: 1.1 Experiment on stretching of steel wire by different loads Objectives The learners will be able to demonstrat different types of deformation The learners will be able to calculate of the ratio of linear stress to linear strain The learners will be able to draw the relation between the stress versus the strain Problem The following problem is helping to find the strength of the material as well as it helps to answer the objectives Hypothesis Formulate an hypothesis about the relation ship between the load and the cross sectional area of the steel wire (stress), the length of the steel to the extension of the steel (strain), calculate the Young’s modulus. Equipment Two long thin steel wires Rigid support Different weight One the wires carries a vernier scale Procedure 1) Arrange the steel wires, the load, the vernier scale as shown 2) Put different loads at the place of w B P Q v M P and Q are steel wires V vernier scale Tensile force on Q A w Figure 1.5 Experimental arrangement for stretching of steel wire by different loads 3. P,Q are two long thin steel wires suspended beside each other from a rigid support B 4. The wire P is kept taut by a weight A attached to its end and carries a scale M graduated in millimeters 5. The wire Q carries a vernier scale v alongside the scale M 6. V measures the small extension e, or change in length of Q, when the load w is increased, and this in turn increases the force F in the wire. Questions 1. What do you observe 2. Calculate the stress 3. Calculate the strain 4. Plot the graph of the stress versus strain Task: 1.2 Experiment to exercise mathematical equations Objectives The learners will be able to derive the mathematical equations for solving problems on coefficient of elasticity problem Derive the mathematical quation on elasticity for the following constants. i)Young’s modulus (E) ii)Bulk modulus(k) iii)Bulk regidity(n) Advise If you have derived the mathematical equations that is very nice. If not please check what is done in derivation Formative evaluation 1 stress strain Fig 1.6 Graph of stress against strain Problem 1 In this activity you are expected to show on for the graph of stress vs strain the following a) elastic range b) elastic limit c) plastic range Answer a) red b) broken line c) red region Problem 2 Mention factors affecting Elasticity Answer Effect of hammering-rolling and a annealingEffect of impuritiesEffect of change of temperature Problems 3 1. Show that a) A small and uniform strain on volume V is equivalent to three linear strain each of magnitude v/3, in any three perpendicular? Answer Imagine a unit cube to be compressed equally and uniformly on all sides, so that length of each edge decreases by a length l and its volume by a small amount v. v Then, clearly volume strain in the cube = = v , and linear strain a long each edge of V l the cube =l L Since length of each edge of the cube now becomes L l the new volume of the cube becomes L l 3 Decrease in volume of the cube, i.e v V L l 3 After calculating and negelecting the higher order of you can find v = 3l Then l v 3 Formative evaluation Show that The bulk modulus for a gas i) at constant temperature (i.e. under isothermal conditions) is equal to its pressure ii) when temperature is not constant, (i.e. when the conditions are Cp adiabatic) it is equal to times its pressure, where Cv Answer Let p be the pressure and V, the volume of a gas , and let it be compressed by increasing the pressure (p+dp), so that the volume is reduced by dv, and becomes (V-dv) then stress = dF = pressure applied =dp dA volume strain = changeinvolume originalvolume bulk modulus for the gas, i.e. K i) dP V dV If the gas is compressed isothermally, its temperature remains constant, therefore PV const P const V dp const dV V2 Vdp const BulkModulu s K V const K V const p V Then Kp Answer bulk constant equal to the pressure ii) if the gas compressed adiabatically pV const , Cp Cv p CV Differentiating p with respect to V gives dp V 1dVconst V dp const dV V Where V k V dp kBulk , const pV dV pV k p Bulk constant ACTIVITY 2: Fluids You will require 45 hours to complete this activity. In this activity you are guided with a series of readings, Multimedia clips, worked examples and self assessment questions. You are strongly advised to go through the activities and consult all the compulsory materials and as many as possible among useful links and references. Specific Teaching and Learning Objectives Describe basic properties of fluid (density,pressure) Apply the properties of fluids (archimedes principle) Explain fluid motion (continuity, turbulance, real fluid) Use Bernnoulli’s Equation Summary of the Learning Activity In this activity the learners will describe the pressure in fluids at rest, explain the effects of the buoyant force on a submerged object and the distribution of fluid in a closed conteiner. The pressure P, in a fluid is the force per unit area that the fluid exerts on any surface. The pressure in a fluid varies with depth(h) according to the expression p pa gh where Pa is atmospheric pressure (1.01x105N/m2) and is the density of the fluid, You will state also Pascal’s law and Archimedes’s principle. Fluid dynamics (fluid in motion) can be understood by assuming that the fluid is non viscous and incompressible and that the fluid motion is a steady flow with no turbulence.Using these assumptions,the flow rate through the pipe is a constant That is A1V1=A2V2 .The sum of, kinetic energy per unit volume, and potential energy per unit volume has the same value at all points along a streamline. That is, p 1 2 v gy constant 2 Bernoulli's equation Key Concepts 1. PASCAL’S PRINCIPLE: A change pressure applied to an enclosed fluid is transmitted undiminished to every point of the fluid and the walls of the containing vessel 2. ARCHIMEDS’ PRINCIPLE: Any body completely or partially submerged in a fluid is buoyed up by a force equal to the weight of the fluid displaced by the body.: 3. STREAM LINE: Is the path taken by a fluid particle under steady flow is called a stream. line 4. BERNOULLI’S EQUATION: This equation gives an expresssion that deals with the sum of the pressure, kinetic energy per unit volume, and potential energy per unit volume has the same value at all points along a streamline Introduction to the Activity The knowledge of the existence of electrostatic charge goes back at least as far as the … Detailed Description of the Activity (Main Theoretical Elements) 2.1 States of matter Matter is normally classified as being in one of its states, solid, liquid or gaseous. Often, this classification is extended to include a fourth state referred to as plasma. The fourth state of matter can occur when matter is heated to very high temperatures. Under this condition, one or more electrons surrounding each atom are freed from the nucleus. The resulting substance is a collection of free electrically charged particles: the negatively charged electrons and the positively charged ions. Such an ionized gas with equal amounts of positive and negative charges is called plasma. 2.2 Density and Pressure The density of a substance is defined as its mass per unit volume. m v Specific gravity of a substance is defined as the ratio of its density to the density of water at 4oc, which is 1x103kg/m3 If F is the magnitude of the normal force on the piston and A is the area of the piston, then the pressure, P, of the liquid at the level to which the device has been submerged is defined as the ratio of force to area. F P A F dF P lim A0 A dA The unit of pressure in the SI system is Pascal (Pa) 1Pa 1 N m2 2.3 Variation of pressure with depth Consider a fluid at rest in a container shown in the Figure 2.1 below Fig 2.1: Variation of pressure with depth in a fluid the volume element is at rest, and the force on it. We first note that all points at the same depth have the same pressure. Consider the fluid contained with in an imaginary cylinder of cross-sectional area A and height dy. The upward force on the bottom of the cylinder is PA and the down ward force on the top is (P+dP) A. The weight of the cylinder, whose volume is dv, is given by dW gdV gAdy , where is the density of the fluid. Since the cylinder is in equilibrium, the force must add to zero, and so we get F y PA P dPA gAdy dP g dy From this result, we see that an increase in elevation (positive by) corresponds to a decrease is pressure (negative dp). If p1 and p2 are the pressure at the elevations y1 and y2 above the reference level, and If the density is uniform, then integrating P2 y2 P1 y1 dP gdy P2 - P1 = - g ( y2 y1 ) If the vessel is open at the top, then the Pressure at the depth h can be obtained. Taking atmospheric pressure to be Pa = P2, and noting that the depth h = Y2 – Y1, We find that: P Pa gh The absolute pressure P at a depth h below the surface of a liquid open to the atmosphere is greater than atmospheric pressure by an amount gh . P2 Pa h y2 p1=p y1 Fig 2.2. The Pressure P at a depth h below the surface of a liquid open to the atmosphere is given by P Pa gh This result also verifies (i) The pressure is the same at all points having the same elevation. (ii) The pressure is not affected by the shape of the vessel. 2.4 Pascal’s principle A change pressure applied to an enclosed fluid is transmitted undiminished to every point of the fluid and the walls of the containing vessel. Fig. 2.3 A hydraulic press P1 P2 F1 F2 A1 A2 2.5 Pressure Measurements One simple device for measuring pressure is the open-tube monometer shown below. Fig. 2.4 The open – tube manometer One end of a U – shaped tube containing a liquid is open to the atmosphere, and the other end is connected to a system of unknown pressure P. The pressure at point B P Pa gh equals where is the density of fluid. But the pressure at B equals the pressure at A. PA = PB P Pa gh The pressure P is called the absolute pressure while P–Pa is called the gauge pressure. 2.6 Buoyant Forces and Archimedes’ Principle Archimedes’ Principle can be stated as follows: Any body completely or partially submerged in a fluid is buoyed up by a force equal to the weight of the fluid displaced by the body. In other words the magnitude of the buoyant force is equal to the weight of the fluid displaced by the object. W B B = W = f Vg = mg where V is the volume of cube and f is density of fluid, m mass of water, W is the weight of fluid displaced. Case 1: A totally submerged object when an object is totally submerged in a fluid of density f , the upward buoyant force is given by B = f V0 g , Where V0 is the volume of the object. If the object has a density 0 , its weight is equal to W = mg= 0V0 g , and the net force on it is B – W = ( f 0 ) V0 g . Hence the density of the object is less than the density of the fluid, the unsupported object will accelerate upward. If the density of the object is greater than the density of the fluid, the unsupported object will sink. Case II: A floating object Consider an object in static equilibrium floating on a fluid; that is one which is partially submerged. In this case, the upward buoyant force is balanced by the downward weight of the object. If vf is the volume of the fluid displaced by the object, then the buoyant force has a magnitude given by B f Vg . Since the weight of the object is W = mg = 0V0 g , and W = B, we see that f Vg = 0V0 g , or 0 V f V0 2.7 FLUID DYNAMICS When fluid is in motion, its flow can be one of two main types of flow. (i) steady flow which a flow where each particle of the fluid flows a smooth path, and the paths of different particles do not cross each other. (ii) a non-steady or turbulent which is an irregular flow characterized by small whirl pool-like region. STREAM LINES The path taken by a fluid particle under steady flow is called a stream line. A particle at P flows one of thesestreamlines, and its velocity V is tangent to the streamline at each point along its path. 2.8 V . P THE EQUATION OF CONTINUITY Consider a fluid flowing through a pipe of non – uniform cross – sectional area. V2 A2 V1 A1 x1 x2 The particles in the fluid move along the streamlines in steady flow. At all points the velocity of the particles is tangent to the stream line along which it moves. In a small time interval Δt, the fluid at the bottom end of the pipe moves a distance Δx 1 = v1 Δt. If A1 is the cross-sectional area in this region, then the mass contained in the shaded region is Δm1= 1 A1 Δx1 = 1 A1 v1Δt. Similarly, the fluid moves through the upper end of the pipe in the time Δt has a mass Δm2 = 2 A2v2Δt. However, since mass is conserved and because the flow is steady, the mass that crosses A1 in a time t must equal the mass that crosses A2 in the time t. Therefore m1=m1, or 1 A1V1 2 A2V2 This is equation of continuity A1V1 A2V2 The product of the area and the fluid speed at all points along the pipe is a constant. 2.9 BERNOULLI'S EQUATION As a fluid moves through a pipe of varying cross section and elevation, the pressure will change along the pipe. We shall assume that the fluid is incompressible and nonviscous and that it flows in an irrotational and steady manner. V2 P2 A2 V1 P1 A1 y1 x1 x2 y2 Consider the flow through a non-uniform pipe in a time Δt. Therefore the force on the lower end of the fluid is P1A1 where P1 is the pressure at point 1. The work done by this force is W 1=F1Δx1=P1A1Δx1= P1ΔV, where ΔV is the volume of the lower shaded region. In similar manner, the work done on the fluid at the upper end in the time Δt is given W2=F2Δx2=-P2A2 Δx2= -P2 ΔV. This work is negative since the fluid force opposes the displacement. Thus the network done by these forces in the time Δt is w = (P 1-P2) ΔV part of this work goes into changing the kinetic energy of the fluid, and part into changing the gravitational potential energy. If Δm is the mass passing through the pipe in the time Δt, then the change in its kinetic energy is k 1 m v2 2 1 m v1 2 2 2 The change in its potential energy is u mgy2 mgy1 We can apply the work energy theorem in the form w=Δk+Δu to its volume of fluid to give 1 1 2 2 (P1-P2) ΔV= m v 2 m v1 + mgy2 mgy1 2 2 m If we divide each term by ΔV, and recall that the above expression reduces to V 1 1 2 2 (P1-P2) = v 2 v1 + gy2 gy1 2 2 Rearranging terms we get 1 1 2 2 P1+ v1 + gy1 = P2+ v 2 + gy 2 2 2 This is Bernoulli's equation as applied to a non-viscous, incompressible fluid in steady flow. It is often expended as 1 2 P+ v + gy constant 2 Bernoulli's equation says that the sum of the pressure, (p), the kinetic energy per unit 1 volume ( 2 ), and potential energy per unit volume ( gy ) has the same value at all 2 points along a stream line. When the fluid at rest v1=v2=0 and the above equation becomes P1 P2 g y2 y1 gh Which agrees with Bernoulli’s equation Learning Activities Task 2.1. Calculation of speed in fluid flow (a) (b) A water hose 2cm in diameter is used to fill a 20 litre bucket. If it takes 1min to fill the bucket, what is the speed v at which the water leaves the hose? If the diameter of the hose is reduced to 1cm, what will the speed of the water be as it leaves the hose, assuming the same flow rate? Task 2.2. Using Archimedes principle to compare densities (a) A plastic sphere floats in water with 0.5 of its volume submerged. This same sphere floats in oil with 0.4 of its volume submerged. Determine the ratio of densities of the oil and the sphere. (b) A cube of wood whose one of the sides is 20cm has a density of 0.65x10 3 floats on water. i. what is the distance from the top of the cube to the water level? ii. how much lead weight has to be placed on top of the cube so that its top is just level with the water? Task 2.3. Using fluid dynamics equations to solve problems 1. Determine the absolute pressure at the bottom of a lake that is 30m deep. 2. A swimming pool has dimensions 30m X 10m and a flat bottom. When the pool is filled to a depth of 2m with fresh water, what is the total force due to the water on the bottom? On each end? On each side? 3. The spring of the pressure gauge has a force constant of 1000N/m, and the piston has a diameter of 2cm. Find the depth in water for which the spring is compressed by 0.5cm? Task 2.4 Using fluid dynamics equations to solve The open vertical tube in the figure shown below contains two fluids of densities 1 And 2 , which do not mix. Show that the pressure at the depth h 1 +h2 is given by the expression P = Pa+ 1 gh1 + 2 gh2 Formative Evaluation 2 1. The rate of flow of water through a horizontal pipe is 2m3/min. Determine the velocity of flow at a point where the diameter of the pipe is (a) 10cm (b) 5cm 2. What is the hydrostatic force on the back of Grand Coulee Dam if the water in the eservoir is 150m deep and width of the dam is 1200m? 3. Calculate the buoyant force on a solid object made of copper and having a volume of 0.2m3 if it is submerged in water. What is the result if the object is made of steel? 4. 2.In air an object weighs 15N. When immersed in water, the same object weighs 12N. When immersed in another liquid, it weights13N. Find a. The density of the object and b. The density of the other liquid ACTIVITY 3: Transport Properties You will require 25 hours to complete this activity. In this activity you are guided with a series of readings, Multimedia clips, worked examples and self assessment questions.. You are strongly advices to go through the activities and consult all the compulsory materials and as many as possible among useful links and references. Specific Teaching and Learning Objectives Analyse particle motion in fluids Describe relative properties of solids, liquids and gasses Discuss the effects of heat on materials – e.g. calculate thermal expansion Calculate the effective concentration of mobile electrons in metals, alloys and semiconductors Summary of the Learning Activity In this unit you will learn the transport properties of gases (molecules) in a system by considering that diffusion, viscocity and heat conduction as a transport process. In addition you will in detailed describtion of conduction and thermal expansion of metals using mathematical approach. The transportaion of electron is discussed in terms of the effective concentration of mobile electrons in metals, alloys and and semiconductors Key Concepts Diffusion: Is the movement of particles from higher chemical potential to lower chemical potential (chemical potential can in most cases of diffusion be represented by a change in concentration).An electric charge is an attribute of matter that produces a force. Osmosis: If two solutions of different concentration are separated by a semi-permeable membrane which is permeable to the smaller solvent molecules but not to the larger solute molecules, then the solvent will tend to diffusion across the membrane from the less concentrated to the more concentrated solution this process is called osmosis. Electron diffusion: resulting in electric Heat Conduction: The conduction of heat is also a process of diffusion in which random thermal energy is transferred from a hotter region to a colder one without bulk movement of the molecules themselves. Viscous motion: of fluids can be far more complicated than diffusion or heat conduction and we will be forced to consider only the steady state equation. Thermal expansion of solids or a body: Is a consequence of the change in the average separation between its constituent atoms or molecules Electrical conductivity: Is the ability of different types of matter to conduct an electric current Semiconductors: are materials whose conductivity is between that of conductors (generally metals) and that of nonconductors or insulators. Alloy: Is a metal composed of more than one element Key terms Momentum diffusion Heat flow Brownian motion Osmosis Diffusion equation Osmotic pressure Fick’s law of diffusion Transport phenomena List of Relevant Readings 1. Reference:- Viscosity Abstract: Viscosity is the resistance or the internal friction between molecules. Viscosity can be measured by an instrument called a viscometer. Some liquids like water have a low viscosity whereas other liquids like honey have a high viscosity. Viscosity will be affected by the temperature. At higher temperatures the viscosity decreases as the molecules take on more kinetic energy allowing them to move past each other faster List of Relevant Resources 1. Reference:- http://video.google.com/videoplay?docid=4559185597114887235&q=electric+charge&hl=en Summary: This resource is video show on electric charges Rationale: 2. Reference: - …http://en.wikipedia.org/wiki/Electrical_conductivity 2. Summary:- To analyse the conductivity of materials exposed to alternating electric field Rationale:- Introduction to the Activity Diffusion is the transport of a material or chemical by molecular motion. If molecules of a chemical are present in an apparently motionless fluid, they will exhibit microscopic erratic motions due to being randomly struck by other molecules in the fluid. Individual particles or molecules will follow paths sometimes known as "random walks." In such processes, a chemical initially concentrated in one area will disperse. That is, there will be a net transport of that chemical from regions of high concentration to regions of low concentration. An analogous form of diffusion is called conduction. In this case, heat is the "chemical" that is transported by molecular motion. As in chemical diffusion, heat migrates from regions of high heat to regions of low heat. The mathematics describing both conduction and diffusion is the same. Figure(3.1 ) Consider two containers of gas A and B separated by a partition. The molecules of both gases are in constant motion and make numerous collisions with the partition Detailed Description of The Activity (Main Theoretical Elements) 3.1: Gases Liquids and Solids As a useful, though not complete, classification it can be said that matter exists in three states, as gas, liquid or solids. This statement is justified by the fact that there exist many substances which can undergo sharp, easily identifiable, reproducible and reversible transitions from one state to the other. Water is the classical example: its freezing and melting, boiling and condensation have been contemplated since the time of the ancient Greek scientists. There are obvious contrast between the properties of ice, water and steam or water vapour which make their description as solid, liquid and gas quite unambiguous. Similarly, most metals are solid, they melt under well defined conditions of temperature and pressure to form liquids and boil at higher temperatures to produce gases. If all substances possessed such clear demarcations, it would be easy to define the different states of matter. But there are very many substances like glasses or glues which one normally thinks of as being solid but which do not melt at sharply defined temperatures; when heated they gradually become plastic, till they become recognizably liquid. Other solids such as wood or stone are inhomogeneous and it is difficult to describe their structure in detail. 3.2: Prosperities and structures of gases Gases have low densities they are highly compressible over wide ranges of volume, they have no rigidity and low viscosities. The molecules are usually a large distance apart compared with their diameter and there is no regularity in their arrangement in space. Given the positions of two or three molecules, it is not possible to predict where a further one will be found with any precision. The molecules are distributed at random throughout the whole volume. The low density can be readily understood in terms of the comparatively small number of molecules per unit volume. The high compressibility follows from the fact that the average distance between molecules can be altered over wide limits. The molecules can move long distances without encountering one another, so there is little resistance to motion of any kind, which is the basis of the explanation of the low viscosity. 3.3 Properties and structure of liquids Liquids have much higher densities than gases and their compressibility is low. They have no rigidity but their viscosity is greater than that of ordinary gases. The molecules are packed quite closely together and each molecule is bonded to a number of neighbors but still the pattern as a whole is a disordered one. The molecules are moving with just the same order of velocity as in a gas at the same temperature, though the motion is now partly in the form of rapid vibrations and partly translational. 3.4. Properties and structure of solids Solids have practically the same densities and compressibilities as liquids. In addition they are rigid; under the action of small forces they do not easily change their shape. An important property of those solids which have a well-defined melting – point is that they are close packed, and the arrangement is highly regular. Substances which do not melt sharply but show a gradual transition to the liquid when heated are said to be amorphous and show no trace of regularity of external shape. In crystalline solids, the molecules are arranged in regular three dimensional patterns or lattices, If the crystal has been carefully prepared, the regular arrangement persists over distances of several thousand molecules in any direction before there is an irregularity, but if it has been subjected to strains or distortions the regular arrangement may be perfect and uninterrupted only over much shorter average distances. In metals the ions are closely packed together, so that the distance between the centre of an ion and that of one of its nearest neighbours is equal to the diameter of one ion, or something close to it. In other crystals, the packing together of the molecules may be relatively open, but even in light solids such as ice the distance between the centers of any molecule and its near neighbors in only twice the diameter of a molecule. In solids, the molecules are again moving with the same order of magnitude of velocity as in gases or liquids, but the motion is confined to vibrations about their mean positions. 3.5. Transport Processes So far we have learned the properties of solids, liquids and gases which are in equilibrium. In this activity we will deal with systems which are nearly but not quite in equilibrium in which the density (or the temperature or the average momentum) of the molecules varies from place to place. Under these circumstances there is a tendency for the non-uniformities to die away through the movement the transport of molecules down the gradient of concentration (or of their mean energy down the temperature gradient or their mean momentum down the velocity gradient). 3.6 Diffusion Diffusion is the movement of molecules from a region where the concentration is high to one were it is lower, so as to reduce concentration gradients. This process can take place in solids, liquids and gases (though this part you will be mostly concerned with gases). Diffusion is quite independent of any bulk movements such as winds or convection currents or other kinds of disturbance brought about by differences of density or pressure or temperature (although in practice these often mask effects are due to diffusion). One gas can diffuse through another when both densities are equal. For example, carbon monoxide and nitrogen both have the same molecular weight, 28, so that there is no tendency for one or other gas to rise or fall because of density differences: yet they diffuse through each other. Diffusion can also take place when a layer of the denser of two fluids is initially below a layer of the lighter so that the diffusion has to take place against gravity. Thus, if a layer of nitrogen is below a layer of hydrogen, a heavy stratum below a light one, then after a time it is possible to detect some hydrogen at the bottom and some nitrogen at the top, and after a very long time both layers will be practically uniform in concentration. Diffusion coefficients of gases and can be measured with a suitable geometrical arrangement of two vessels with different initial concentrations together with some method of measuring those concentrations such as a chemical method or mass spectroscopy, for example. If the rates of change of concentration with time are plotted, the diffusion coefficient can be deduced; the equations describing the process are given in diffusion equation. t= 1/4D concentrat ion T= 1/2D T=1/D Fig 3.2 Concentration as a function of x for different values of time t 3.7. The diffusion equation We will begin by taking a macroscopic view of the phenomenon, that is, we will write down equations which involve such variables as concentrations or fluxes but will not specifically mention individual molecules. We define the concentration as the number of molecules n per unit volume. Let us consider the simple case where n varies with one coordinate only the x-axis. In Figure 3.1 the concentration at all points in the plane x is n, at (x+dx) it is (n+dn). Then diffusion takes place down the concentration gradient, from high to low concentration; we are assuming that bulk disturbances are absent. We next define the flux J of particles as the number of particles on average crossing unit area per second in the direction of increasing x. Notice that both concentration and flux can be measured in moles instead of numbers of molecules: this is equivalent to dividing all through our equations by Avogadro’s number N. n n+dn J X X+dX X Fig 3.3 Coordinates used in the definition of diffusion In general, the flux J may change with position x and may also change with time t. In other words, J may be a function of x and t so we write it as J (x,t). Of course, there are circumstances where J may be the same for all x, or where it is constant with time, but the most general situation is that j depends on both. It is an experimental fact that, at any instant that flux at any position x is proportional to the concentration gradient there: n J x, t or ………………………3.1 x n J x, t D x where D is called the diffusion coefficient. This is known as Fick’s law. By itself, Eq. (3.1) is adequate to describe ‘steady-state’ conditions where currents and concentrations do not change with time so that the flux can be written J(x). For example, if a tube of length l cm with constant cross-sectional area A cm2 has molecules continually introduced at one end and extracted at the other end at the same rate, the concentration gradient becomes -n/ l , where n is the difference of concentration between the two ends. The number of particles crossing any plane in the tube per second is then –DAn/ l and this does not change with time. Consider, however, the much more general situation where initially a certain distribution of concentration is set up and then subsequently the molecules diffuse so as to try to reach a uniform concentration. Concentrations are, therefore, changing with time and particles must be accumulating in the region between x0 and (x0+dx) or moving from it. Therefore, the number crossing area A of the plane x0 is not equal to that crossing the same area at (x0+dx). The flux entering this volume is n Jx0 = - D x x x0 The flux leaving the slice can be written Jx0+dx where J dx ... Jx0+dx = Jx0+ x that is and we can neglect higher terms. The rate of movement of molecules from the slice is equal to the difference between the two values of AJ, and also equal to the volume of the slice, A dx, times the rate of decrease of n: J n A dx A d t - x J n x t ………………………… (3.2) That is Combining this with equation (3.1) and eliminating J: n n 2n D D 2 t x x x ……………………………………………………. (3.3) if we assume that D is constant independent of the concentration. This is called the diffusion equation, and since n depends on x and t it could be written n(x,t). If the process takes place in 3 dimensions, J is a vector whose components are (Jx,Jy,Jz) and the above equations become n n n J iJ x jJ y kJ z D i j k D grad n y z x n J x J J div J t x y z Where i,j and k unit vectors parallel to x,y and z. Eliminating J: 2n 2n 2n n div ( D grad n) D2 n D 2 2 2 t y z x Thus we have a system of three equations. (3.1) is an experimental law linking the flux at any point with the concentration gradient there. (3.2) is the continuity equation expressing the fact that molecules cannot disappear, and (3.3) combines these two equations. Eq. (3.1) is adequate for steady-state conditions, where conditions do not vary with time; but for the general case (3.3) may be used. These are typical of transport equations with the provision that for energy and momentum diffusion, the coefficients in the three equations are not all identical as they are here. 3.8 Heat conduction Heat can be transferred by conduction, convection or radiation. The process of transferring heat through a body is called thermal conduction. The physical property known as thermal conductivity is a measure of how efficient the material will conduct heat through it. The thermal conductivity of a substance is defined as the amount of heat transfer per unit area per unit time per unit temperature gradient through a body. Mathematically, thermal conductivity can be treated in a very similar way to diffusion leading to very similar types of mathematical functions. Thermal conductivity is very important when designing for thermal insulation, thermal isolation, efficient heat transfer and cooling systems The conduction of heat is also a process of diffusion in which random thermal energy is transferred from a hotter region to a colder one without bulk movement of the molecules themselves. In a hot region of a solid body, they have extra kinetic energy. By a collision process, this energy is shared with and transferred to neighbouring molecules, so that the heat diffuses through the body though the molecules themselves do not migrate. The macroscopic equations describing conduction in one dimension x are, firstly, the experimental law for the heat flux T Q k ………………………………………………………..(3.4) x (where Q is the heat flux across unit area, measured in W cm -2, k is the thermal conductivity and T is the temperature) and, secondly, the continuity equation Q T Cp ……………………..(3.5) x t which expresses the conservation of energy in the form that the heat which is absorbed by a slice of a body goes into raising its temperature. C is the specific heat per unit mass, the density so that C is the specific heat per unit volume. Combining these two equations to eliminate Q: T k 2T t Cp x 2 ………………(3.6) k C where is called the thermal diffusivity by analogy with Eq. (3.3). E1. (3.4) by itself is adequate for steady-state conditions, as when for example heat is fed into one end of a bar and extracted at the other and all temperatures are constant with time, and T can be calculated as a function of x alone. But when conditions are not steady, and T varies with time as well as position, Eq. (3.6) describes the situation. Viscosity For completeness, a third simple transport process the diffusion of momentum by viscous forces will be mentioned here, briefly. Viscous motion of fluids can be far more complicated than diffusion or heat conduction and we will be forced to consider only the steady state equation. Z Moving plate Y Ux X Stationary plate Figure 3.4 Coordinates used in the definition of viscosity. Consider a gas or liquid confined between two parallel plates (Fig.3.4). Let the lower plate be stationary and the upper plate be moving in the direction shown, which we will call the x-direction. Molecules of fluid very near the plate will be dragged along with it and have a drift velocity, Ux parallel to x, superposed on their thermal velocity. We will assume that Ux is much less than the mean thermal speed or the speed of sound. Molecules of fluid near the stationary plate will, however, remain more or less with zero drift velocity. Eventually a regime will be set up in which there is a continuous velocity gradient across the fluid from bottom to top. In this state, molecules will be continuously diffusing across the space between the plates and taking their drift momentum with them. Considering an area of a plane parallel to the xy plane in the fluid, molecules which diffuse across from above to below will carry more drift momentum than those which diffuse from underneath to the top. In other words, the more rapidly moving layer tends to drag a more slowly moving layer with it, because of this diffusion of momentum. In macroscopic terms, a shearing stress (force per unit area) is necessary to maintain this state of motion. The experimental law is U x .. ……………..(3.7) z where Pxz is the force per unit area in the x direction due to a gradient of U x in the zdirection and is called the coefficient of viscosity. Provided the direction of the force is clearly understood, it is not necessary to include a minus sign, as this depends on the convention for the choice of axes. Pxz We started by considering a fluid in Figure 3.4, but Eq. (3.7) can be applied to solids d because the right-hand side can be written dt , where is an angle of shear. It is difficult to imagine a solid subjected to a shear which goes on increasing with time, but it is quite common for solids to be sheared to and for in an oscillatory fashion. Forces are then required to provide the accelerations, but in any case the viscosity gives rise to the dissipation of energy and the production of heat. It is usual to refer to this as due to the internal friction of solids. U x is constant and that Ux increases proportionally with z. z This is so if the coefficient is a constant. For many liquids this holds, but there are notable exceptions when varies with the velocity gradient or rate of shear so that the velocity profile is not linear It is implied in Figure.3.4 that When we come to write down equations representing the motion of a fluid while it is not in a steady state but accelerating, we meet a situation which is much more complicated than the diffusion or heat conduction cases. For one thing, there are always massacceleration terms which have no analogue in the other phenomena. For another, a kind of regime may be set up where the flow is not streamline as illustrated in Figure. 3.4 but turbulent, and vortices or eddies are present which add an element of randomness to the flow pattern. We can, however, usefully adopt a mathematical representation of the simple situation of Fig3.4. We can imagine the liquid divided into layers, each one sliding over the one underneath it on imaginary rollers like long axle rods parallel to the y-axis. These rollers are not there in any real sense, but they can lead one to define a quantity called the vorticity which is always present in a flowing fluid even when no macroscopic vortices are present. (In a simple case like Fig.3.4 the vorticity degenerates into the velocity gradient.) Now in the general case of an accelerating fluid with non-uniform velocity it is the vorticity which diffuses throughout the fluid, though the equation it obeys is not of a simple form Task 3.1 Measurement of the viscosity of gases In his classic experiments to measure the viscosity of gases at low pressures, Maxwell used a torsion apparatus in which a number of circular glass discs were arranged to swing in between fixed ones (Fig.3.5.). He found the damping coefficient of the oscillations. If we neglect the energy loss in the torsion wire itself and assume that the discs would go on swinging for a very long time if all the gas were removed, we can calculate the damping as follows. Fig. 3.5 Principle of the apparatus for measurement of viscosity by the damping of torsional oscillation. Consider one surface of one plate, and select an annulus ring between radii r and (r+dr). Then (assuming streamline flow) the force on this annulus ring, whose area is 2 dr, is r (2rdr ) dF= d ………3.8 Where the linear velocity is r , being the angular velocity, and d is the spacing between adjacent moving and stationary surfaces. The contribution to the couple is the couple is the radius times the force: 2 3 r dr dG= d ………..3.9 and the total couple is 2 a 3 4 r dr a 0 2d G= d ………..3.10 Where a is the radius of the disc. If there are n discs, each with two surfaces, there are 2n such contributions. t LAW Consider fiure3.3 coordinates used in the definition of diffusion the length is along the xaxis and the ends are at x=0 and x =. On the face x=0,N0 molecules are initially all concentrated in a thin layer and are subsequently allowed to diffuse into the material. We will denote the number at time t which are within a slice between x and (x+dx) by n (x,t) A dx. Then the appropriate solution of Eq.(3.3) shows that the concentration. x2 N0 4 Dt n x, t e 1 ADt 2 ……………. 3.11 SOLUTIONS OF THE DIFFUSION EQUATION: THE We can, therefore calculate the mean net distance traveled by a molecule at any time t. A xt xnx, t dx N 0 0 1 2 Dt 2 x we find We find the mean net distance traveled is proportional to the square root of the time. This is perhaps an unexpected result: one is used to traveling twice the distance when the time is doubled, but for the random process of diffusion this is not so. Of course, some molecules go much further than this, other less far, and it is the mean which we have calculated. Stated differently , our results shows that to diffuse a mean distance. X, the time required is proportional to x2 . This is an important characteristic of the diffusion process. 3.9 Thermal Expansions of solids and liquids. Most solids expand as their temperature increases. The thermal expansion of solids or a body is a consequence of the change in the average separation between its constituent atoms or molecules. Suppose the linear dimension of the body along some direction is at some temperature. The length increases by an amount for a change in temperature T l l0 Then T = T = T Where is coefficient of linear expansions of solids? The linear dimension of the body also change with temperature, it follows that area and volume of a body also change with temperature. l V V0 T 3 is the coefficient of volume expansion = 3 for isotopic solid where the coefficient of linear expansion is the same in all direction. For a side of volume , , V+ V = ( ) ( ) ( ) = ( 2T ) ( T ) ( T ) = (1 T ) (1 T ) (1 T ) = (1 T ) 3 = ( 3 T ) 3(T ) 2 + (T )3 = V (1 3T ) 3 ( T ) 2 + ( T ) 2 Comparing (T )3 << T T 2 << T Then we neglect T 3 and T 2 V+ V =[ 1+3 T 3 ( T 2 ) + ( T 3 )] V = [V 3 T 3 A V 3T V 3 = V T For a flat plate A 2 AT 2 A AT (T ) 2 + (T )3 ] 3.10 Electrical conductivity Electrical conductivity is the ability of different types of matter to conduct an electric current. The electrical conductivity of a material is defined as the ratio of the current per unit cross-sectional area to the electric field producing the current. Electrical conductivity is an intrinsic property of a substance, dependent on the temperature and chemical composition, but not on the amount or shape. Electrical conductivity is the inverse quantity to electrical resistivity. For any object conducting electricity, one can define the resistance in ohms as the ratio of the electrical potential difference applied to the object to current passing through it in amperes. For a cylindrical sample of known length and cross-sectional area, the resistivity is obtained by dividing the measured resistance by the length and then multiplying by the area. The conductivity () of a material is determined by taking the reciprocal of the measured electrical resistance (R) to the flow of electricity in a length (L) of material divided by the 1L cross-sectional area (A). . R A Conductivity is temperature dependent. T ' T 1 (T T ') where σT′ is the electrical conductivity at a common temperature, T′ σT is the electrical conductivity at a measured temperature, T α is the temperature compensation slope of the material, T is the measured temperature, T′ is the common temperature Metals generally have very high electrical conductivity. The electrical conductivity of copper at room temperature, for instance, is over 70 million siemens per meter. On an atomic level this high conductivity reflects the unique character of the metallic bond in which pairs of electrons are shared not between pairs of atoms, but among all the atoms in the metal, and are thus free to move over large distances. Many metals undergo a transition at low temperatures to a superconducting state, in which the resistance disappears entirely and the conductivity becomes infinite. The superconduction process involves a coupling of electron motion with the vibration of the atomic nuclei and innershell electrons, to allow net current flow without energy loss. Electrical conductivity in the liquid state is generally due to the presence of ions. Substances that give rise to ionic conduction when dissolved are called electrolytes. The conductivity of one molar electrolyte is of the order of 0.01 siemens per meter, far less than that of a metal, but still very much larger than that of typical insulators. Sodium chloride (common table salt), composed of sodium ions and chloride ions, is a very poor conductor in the solid state. If it is dissolved in water, however, it becomes a good ionic conductor. Likewise, if it is melted, it becomes a good conductor. Substances such as hydrogen chloride or acetic acid are non-conductors in the pure state but give rise to ions and thus electrical conductivity when dissolved in water. In modern electrochemistry, substances of the sodium chloride type, which are actually composed of ions, are termed true electrolytes, while those that require a solvent for ion formation, like hydrogen chloride, are termed potential electrolytes. The unit of electrical conductivity in the International System of Units (SI) system is the siemens per meter, where the siemens is the reciprocal of the ohm, the unit of electrical resistance, represented by the Greek capital letter omega ( ). An older name for the siemens is the mho, which, of course, is ohm spelled backwards (which was written as an inverted Greek omega). Semiconductors are materials which have a conductivity between conductors (generally metals) and nonconductors or insulators (such as most ceramics). Semiconductors can be pure elements, such as sillicon or germanium, or compounds such as gallium arsenide or cadmium selenide. In a process called doping, small amounts of impurities are added to pure semiconductors causing large changes in the conductivity of the material. Metals and alloys An alloy is a metal composed of more than one element. Engineering alloys include the cast-irons and steels, aluminum alloys, magnesium alloys, titanium alloys, nickel alloys, zinc alloys and copper alloys. For example, brass is an alloy of copper and zinc. This versatile construction material has several characteristics, or properties, that we consider metallic: (1) It is strong and can be readily formed into practical shapes. (2) Its extensive, permanent deformability, or ductility, is an important asset in permitting small amounts of yielding to sudden and severe loads. Many Californians have been able to observe moderate earthquake activity that leaves windows (of relatively brittle glass) cracked while steel support framing still functions normally. (3) A freshly cut steel surface has a characteristic metallic luster, and (4) a steel bar shares a fundamental characteristic with other metals: it is a good conductor of electrical current. Although structural steel is a special common example of metals for engineering, a little thought produces numerous others [such as gold, platinum, lead and tin]. Learning Activities Task 3.1. The mean distance travelled by a molecule at any time t. Calculate the mean distance travelled by a molecule at any time t n x, t N0 ADt use e x dx 2 0 solution 1 2 e x2 4 Dt 1 2 diffusion equation x 2 1 ( Dt ) 2 Task 3.2: Derive the surface and volume expansion coefficients a) For volume expasion show that V V T 3 b) For a flat plate show that A AT 2 Task 3.1 Problem 1. Consider a composite structure shown on below. Conductivities of the layer are: k1 = k3 = 10 W/mK, k3 = 16 W/mK, and k4 = 46 W/mK. The convection coefficient on the right side of the composite is 30 W/m2K. Calculate the total resistance and the heat flow through the composite. 2. An aluminum tube is 3m long at 200C. What is its length at 1000C. A metal rod made of some alloy is to be used as a thermometer. At 00C its length is 40cm, and at 1000C its length is 40.06cm. a. What is the linear expansion coefficient of the alloy? b. What is the temperature when its length is 39.975cm? 4. At 200C, an aluminum ring has an inner diameter of 5cm, and a brass rod has a diameter of 5.05cm. a. To what temperature must the aluminum ring be heated so that it will just slip over the brass rod? b. To what temperature must both be heated so the aluminum ring will slip off the brass rod? Would this work? V 5. Calculate the fractional change in the volume ( ) of an aluminum bar that undergoes a V change in temperature of 300C 3. Solution 1. First, draw the thermal circuit for the composite. The circuit must span between the two known temperatures; that is, T1 and T∞. Next, the thermal resistances corresponding to each layer are calculated: Similarly, R2 = 0.09, R3 = 0.15, and R4 = 0.36 To find the total resistance, an equivalent resistance for layers 1, 2, and 3 is found first. These three layers are combined in series: The equivalent resistor R1,2,3 is in parallel with R4: Finally, R1,2,3,4 is in series with R5. The total resistance of the circuit is: Total thermal resistance Rtotal = R1,2,3,4 + R5 = 0.46 The heat transfer composite is: through the = 173.9 W. ← heat flow through the composite Formative Evaluation 3 1.What is the properties of semiconductor a) it is an in sulators b) it is con ductors C it is material which has a conductivity between conductors (generally metals) and nonconductors or insulators 2. The hollw cylinder as shown in the figure has the length L and inner and outer radii a and b. It is made of a material with resistivity . A potential difference is set up between the inner and outer surface of the cylinder so that current flow radially through the cylinder. What is yhe resistance to this radial current flow b. a Solution dr dR 2 rL b dr 2 L a r b R ln 2 L a R 3 Derive the diffusion equation in 1D 3. State the properties of solid ,liquid and gas XV SYNTHESIS OF THE MODULE Electricity and magnetism I needs your expertise. EXPECTED SOLUTIONS TO SOME PROBLEMS SET XVI. Summative Evaluation Summative evaluation 1. determine Youn’s modulus, Bulk modulus and Poisson’s ratio and derive a relation between them 2. Asteel wire 2mm in diameter is just stretched between two fixed points at a temperature of 200C. Determine its tension when the temperature falls to 10 0C. (coefficient of linear expansion of steel is 0.000011 and Young’s modulus for steel is 2.1x1012dynes per sq.cm) Solution let the length of wire be Lcm then, on a fall in temperature, from 200C to 100C, its length will decrease by an amount L LT = L90.000011)(10) L ( L)11x10 5 L L =(L)(11x10-5)/L =11x10-5 -the strain produced in it -strees =T/ r2 =T/ (0.1) 2 stress strain =7.3x106dyne. Young’s moduolus (Y) = 3. Define stress, strain and Young’s modulus. 4. A copper wire 3 meters long of Young’s modulus 2.5x1011dyne/cm2 has a diameter of 1mm. If a weight of 10kg is attached to one end what extension is produced? If poisson’s ratio is 0.26, what lateral compression is produced? Solution Original length of the wire (L)=3m Young’s modulus for the wire (Y)=12.5x1011dynes/cm2 Radius of the wire (r)= ½ mm Its area of cross section= r2 Force applied (F)= 10kgmwt.= 981x104 dyne From the relation F .L F .L Y= , then l = 0.2997cm a.Y a.l lateralstrain Poisson’s ratio, longitudin alstrain lateralstr ain 0.26= l L Lateral strain = 0.26x l = 2.6x10-4 L This, therefore, gives the value of lateral strain, i.e, d/D, where d is the decrease in diameter (d/D) = 2.6x10-4 d = D(2.6x10-4) = 2.6x10-5cm is lateral compression 5. Establish an expression for the workdone in streching a wire through 1cm, assuming Hooke’s law to hold. Find the work done in joules in stretching a wire of cross-section 1sq.mm and length 2meters through 0.1mm, if young’s modulus for the materials of the wire is 2x1012dynes/cm2 Solution Work done =(1/2) stretching x the stretch = (1/2) F. l = ½ .(Y.a)/L . l =5x10-4 joule 6. Show that the bulk modulus k, Young’s moduous E and the Poisson’s ratio are E connected together by the relation k 3 1 2 Solution We have k 1 then 3 2 Therefore k 1 3 1 2 where E 1 and Y 31 2 7. show that the rigidity n, and young’s modulus E are connected by te relation 1 where is the poisson ratio n 21 solution 1 2 1 n 2 1 1 But Y 1 Therefore n 21 we have n 8. Water flows along a horizontal pipe, whose cross- section is not costant. The pressure is in cm/sec. Find the pressure at a point where the velocity is 65cm/s. solution p1=1cm=1 x 13.6 x 981 dynes/cm2 V1= 35cm/s, V2 = 65 cm/s, = 1 gm/cm3 P2 =? Appling Bernoullis relation 1 1 2 2 P1 – P2 = V1 V2 2 2 1 2 2 = V1 V2 2 P2= 0.89cm of mercury 9. Define the coefficient of viscosity. Give examples of some viscous substances. How would you determine the coefficient of a liquid? 10. State a) the law of fluid pressure b) The principle of Archimedes 11. A string supports a solid iron object of mass 180gm totally immersed in a liquid of density 800kg m-3. Calculate the tension in the string if the density of iron is 8000kg/m3 solution The tension in the string = weight of an object in the air – the weight of liquid displaced T= Mg-mg where m=(.18/8000) x 800 =18gm =(0.18 x 10 - 0.018 x 10 ) =(1.8 - .18 ) =1.62N 12. At 200C, an aluminum ring has an inner diameter of 5cm, and a brass rod has a diameter of 5.05cm. a) To what temperature must the aluminum ring be heated so that it will just slip over the brass rod? To what temperature must both be heated so the aluminum ring will slip off the brass rod? Would this work? XVII. References 1. Finn, C. B.P (1993). Thermal Physics , Chapman & Hall, London. 2. Raymond A. Serway (1992). PHYSICS for Scientists & Engineers. Updated Version. 3. Kleppner & Kolenkow An introduction to mechanics. 4. Douglas D. C. Giancoli Physics for Scientists and Engineers. Vol. 2. Prentice Hall. 5. Sears, Zemansky and Young, College Physics, 5th ed. 6. Sena L.A. (1988) Collection of Questions and Problems in Physics, Mir Publishers Moscow. 7. Nelkon & Parker (1995), Advanced Level Physics, 7th ed, CBS Publishers & Ditributer, 11, Daryaganji New Delhi (110002) India. ISBN 81-239-0400-2. 8. Godman A, and Payne E.M.F, (1981) Longman Dictionary of Scientific Usage. Second Impression, ISBN 0 582 52587 X, Commonwealth Printing press Ltd, Hong Kong. 9. Siegel R. and Howell J. R., (1992) Thermal Radiation Heat Transfer, 3rd ed., Hemisphere Publishing Corp., Washington, DC. 10. Kittel C. and Kroemer H., (1980) Thermal Physics, 2nd ed., W. H. Freeman and Co., San Francisco, CA. 11. Zemansky M. W. and Dittman R. H., (1981) Heat and Thermodynamics, 6th ed., McGraw Hill Book Co.. 12. Halliday D., Resnick R., and Walker J. (1997), Fundamentals of Physics, 5th ed., John Wiley and Sons XIX. Main Author of the Module About the author of this module: Name:Sisay Shewamare Address: Department of physics, Jimma University, Ethiopia, East Africa. P.O.Box (personal), (Institutional) E-mail : sisayshewa20@yahoo.com Tel: +251-91-7804396 Brief Biography: My name is Sisay Shewamare I am living in Ethiopia I am working in Jimma university department of physics. You are always welcome to communicate with the author regarding any question, opinion, suggestions, etc about this module. Thank you






