Atoms and Molecules Practice Problems
advertisement

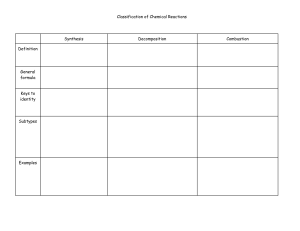
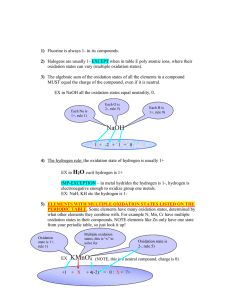
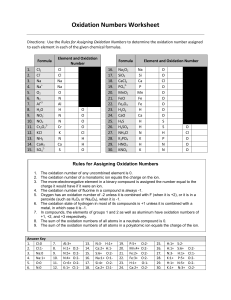
Practice Problems! Use your copy of the periodic table to determine the number of Protons, Neutrons, and Electrons in each of the following atoms 1. Lithium 2 Iron 3. Arsenic 4. Mercury P _______ P _______ P ______ P ______ N ______ N ______ N _____ N _____ E _______ E _______ E ______ E ______ Look at the chemical formulas listed below. Using your periodic table and your knowledge of oxidation numbers, list the elements present and the oxidation numbers for each. Identify the sum of the oxidation numbers for the compound and identify if the compound would be stable (happy) or unstable (unhappy). Chemical Formula (Compound) Oxidation Numbers for each element Sum of Oxidation Numbers Happy or Unhappy? CaO LiI Al2S3 Using your periodic table, draw the electron shell model (Bohr model) for each of the elements listed. (Use a separate piece of paper) Also, write the name of ONE element that could combine with that atom to form a happy, stable compound. (Hint: Remember your oxidation numbers) - Beryllium Phosphorus Carbon Potassium