Acid/Base Review
advertisement

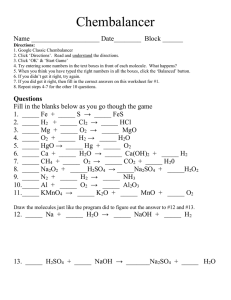
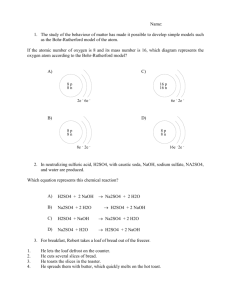
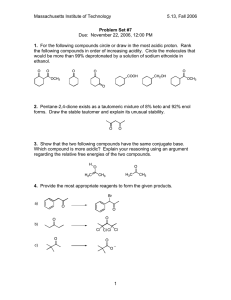
Acid/Base Review 1. Compare acids and bases in terms of the following properties: taste, touch, reactions with metals, reactions with litmus paper and electrical conductivity. 2. Name the following acids/ bases or write the formulas a. HF b. H2SO4 c. HNO2 d. LiOH e. Mg(OH)2 f. ammonium hydroxide g. oxalic acid h. hydrosulfuric acid i. nitrous acid 3. Hydrofluoric acid (HF) is a weak acid. If the initial concentration of the acid is 0.11 M and at equilibrium, [H+] = 3.4x10-2 M, what is the Ka of the acid? 4. Gallic acid is a weak monoprotic acid. If the initial concentration of gallic acid is 0.28 M and at equilibrium, pH of 2.48, calculate the Ka for gallic acid. 5. The Ka for nitrous acid is 5.1x10-4 M. What is the [H+] at equilibrium for a 0.25 M nitrous acid solution? 6. State Arrhenius’ definition of an acid and base. 7. State Bronsted-Lowry’s definition of an acid and base, and why did this pair of chemists change Arrhenius’ acid-base definition? 8. Using Brosted-Lowry’s definition, identify the acid and base and its conjugate pairs: a. H2O + NH3 → NH4+ + OHb. H2O + HCl → H3O+ + Cl- c. H2SO4 + H2O → H3O+1 + HSO4-1 d. C6H5NH2 + H2O → C6H5NH3+1 + OH-1 9. What does the pH of a solution measure and indicate? 10. What pH values correspond to an acid? A base? A neutral solution? 11. What numerical value determines the strength of an acid or base? 12. During titration, what is the essential player that tells you once you have reached neutralization? 13. 50 mL of KOH solution are neutralized by 75 mL of a 2.0 M HBr solution. What is the concentration of the base? 14. How many mL of a10 M HCl are needed to neutralize 380 mL of a 7 M NaOH? 15. 40 mL of an HF solution are neutralized by 18 mL of an 8 M Ca(OH)2 solution. Find the acid’s concentration. 16. When neutralizing H3PO4 with NaOH, it took 49 mL of a acid to neutralize 74 mL of 0.85 M NaOH solution. What is the concentration of the acid? 17. Rank these acids from weakest to strongest: Oxalic acid (Ka = 5.6x10-2), carbonic acid (Ka = 4.3x10-7), benzoic acid (Ka = 6.3x10-5)