what is a penny worth - School District of Clayton
advertisement

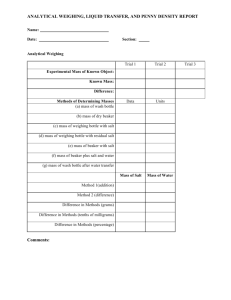
WHAT IS A PENNY WORTH??? (Lab Guidelines: 1,2,3,4,5,6,7,9,10,11,12) Purpose: To determine the actual monetary value of a penny, using your knowledge of chemical reactions and stoichiometry. Introduction: In 1982 the U.S. government made some changes in the composition of the penny. Earlier pennies were composed of over 95% copper. In 1982 the government began producing pennies with a much different compostion. Why was this change made? The cost of copper became so expensive that the penny had to be altered so that producing pennies did not become overly expensive. In this lab you will be using your knowledge of chemical reactions and stoichiometry to determine the actual value of a penny. What do you think a penny is worth? Calculate how many mL of 6.0 M hydrochloric acid you will need to react with 3 pennies. This means you must write the balanced equation for the reaction which will take place and do a stoichiometry problem. Show your answer to your instructor. (Note: You will need to know the approximate mass of 3 pennies to do this calculation). Include this balanced equation and stoichiometry calculation in your introduction. Materials: 250 mL Beaker, Balance, file, 6.0 M hydrochloric Acid, 50 mL graduated cylinder, forceps, scissors, 3 post 1982 pennies Procedure: You decide. Be sure to be specific when writing this portion of your lab report. Also, you should use at least 3 pennies. Remember – the larger the sample size, the smaller your error. Finally, underline the materials as you mention them in your procedure. Data: Don’t forget anything! Create your own data table. Calculations: Determine the actual monetary value of a penny. Show your calculations in an organized manner. Label what you are calculating. Use proper units. Circle answers. Note: the price of copper = $2.59/pound (as of Feb 9, 2015) the price of zinc = $0.97/pound Questions: 1) In this lab what was the limiting reactant? What was the excess reactant? 2) A) What volume of the excess reactant remained unused at the end of the lab? (For this question, you cannot make use of the stoichiometry calculation in the introduction because in that calculation, you assumed that the entire penny was made of zinc. So you need to do a similar calculation, but start with the actual amount of zinc in your three pennies.) B) What is the concentration of the excess reactant after the reaction stops? (Assume a final volume of 50.0 mL.) 3) What was the identity of the metal left in the beaker? Why didn’t this metal react? Please make reference to the activity series, and oxidation and reduction in your answer. 4) A) Calculate the mass of zinc chloride produced in this lab (Combine all three trials together). Why wasn’t this zinc chloride visible in the beaker? B) Now calculate the concentration of the zinc chloride at the end of the reaction (Assume a final volume of 50.0 mL) 5) Calculate the volume of hydrogen gas produced in this lab (Combine all three trials together). 6) Write the net ionic equation for the reaction which took place. 7) Use the price per pound data and the current composition of a penny according to the government (97.5% Zn and 2.5% Cu) to calculate the actual value of a penny. (This will serve as your accepted value for the error analysis) Error Analysis: Use your answer from #7 above and your experimental value of a penny (determined from your calculations), in order to calculate your percent error. Discuss possible sources of error and their impact on your results. Conclusion: Write a conclusion which demonstrates that you understand what you did. How did you accomplish the purposes? Discuss the math as well. Remember, here is a great guide for writing your conclusion: 1) Restate the purpose 2) Summarize the procedure and data collection 3) Discuss any important principles / equations used 4) Explain the graphs and/or math 5) Report your results Please include a discussion of whether or not you think the U.S. should continue to produce pennies.