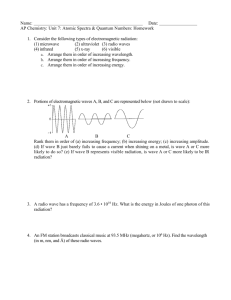
File - MHS Pre
advertisement
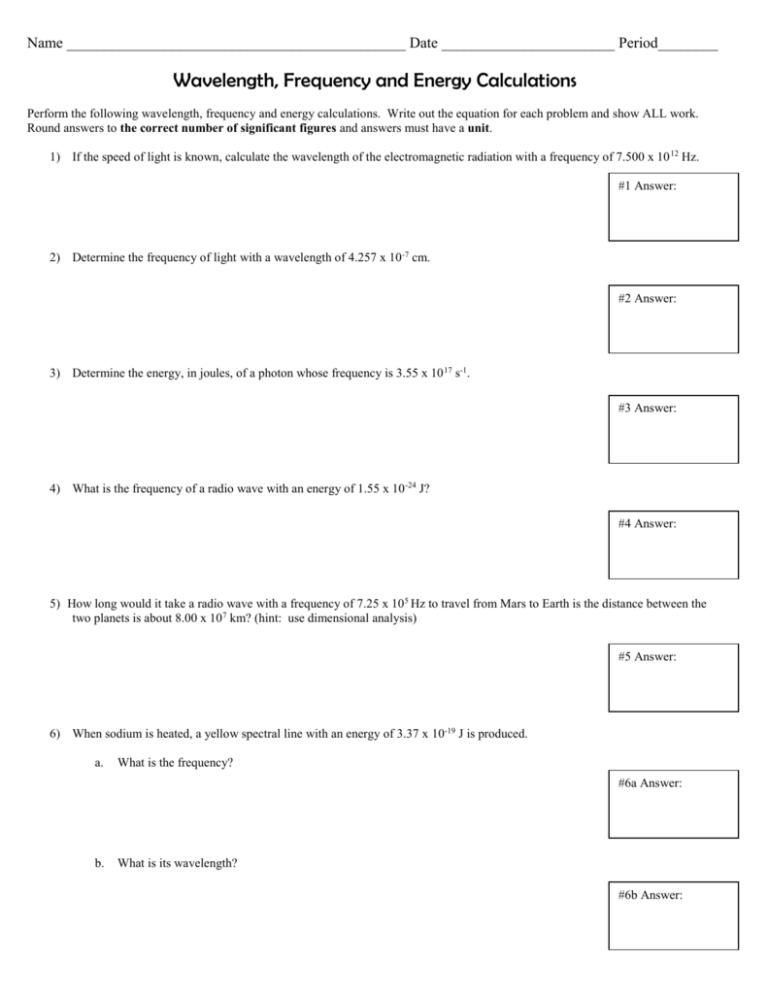
Name _____________________________________________ Date _______________________ Period________ Wavelength, Frequency and Energy Calculations Perform the following wavelength, frequency and energy calculations. Write out the equation for each problem and show ALL work. Round answers to the correct number of significant figures and answers must have a unit. 1) If the speed of light is known, calculate the wavelength of the electromagnetic radiation with a frequency of 7.500 x 10 12 Hz. #1 Answer: 2) Determine the frequency of light with a wavelength of 4.257 x 10 -7 cm. #2 Answer: 3) Determine the energy, in joules, of a photon whose frequency is 3.55 x 10 17 s-1. #3 Answer: 4) What is the frequency of a radio wave with an energy of 1.55 x 10 -24 J? #4 Answer: 5) How long would it take a radio wave with a frequency of 7.25 x 10 5 Hz to travel from Mars to Earth is the distance between the two planets is about 8.00 x 107 km? (hint: use dimensional analysis) #5 Answer: 6) When sodium is heated, a yellow spectral line with an energy of 3.37 x 10-19 J is produced. a. What is the frequency? #6a Answer: b. What is its wavelength? #6b Answer: 7) What is the energy of a quantum of light with a frequency of 4.31 x 10 14 Hz? #7 Answer: 8) What is the energy content of one quantum of violet light with a wavelength of 413 nm? #8 Answer: 9) Calculate the wavelength of the yellow light emitted by a sodium lamp if the frequency of the radiation is 5.10 x 10 14 s-1. #9 Answer: 10) What is the frequency of radiation with a wavelength of 5.00 x 10 -5 cm? #10 Answer: 11) According to the following diagram, which excited electron (A, B, or C) will emit the largest amount of energy when it returns to the ground state? Explain your answer in relationship to energy levels and further support your answer with energy calculations.