Physical Science Final
advertisement

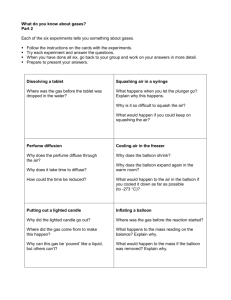
8th Grade Physical Science Assessment DIRECTIONS: 1. Read each question carefully and read all answer choices. 2. Choose the word or phrase that BEST answers each question. 3. Using CAPITAL LETTERS, write the appropriate letter for your answer choice on your answer sheet. 4. PLEASE DO NOT WRITE ON THE TEST PACKET. 1. What is the first step a scientist usually takes to solve a problem? A. Conduct research B. Form a hypothesis C. Gather information D. Identify the problem 2. This is what you think about what you are observing A.Hypothesis C. Observation B. Inference D. Theory 3. A factor that should remain the same in an experiment is the ______. A. Constant C. Independent variable B. Control D. Dependent variable 4. Which of the following values is the same as 3.3 minutes? A.3 minutes, 3 seconds C. 3 minutes, 30 seconds B. 3 minutes, 18 seconds D. 3 minutes, 33 seconds 5.How many millimeters are in 1 meter? A.10 C. 1,000 B. 100 D. 10,000 6. A measurement of the quantity of matter is __________. A. mass C. density B. volume D. temperature Answer questions 7 – 9 using the information below. A student wants to find out if the temperature of the water affects how far her film canister lid launches in the Alkaseltzer cannon. She ran her trials using the same film canister, 15 mL of water each time, and 1 tablet of Alkaseltzer each time. However, she changed the temperature of the water each time as follows: one at room temperature (23°C), 10°C, 30°C, 50°C, 70°C, and 90°C. The following chart shows the results of her experiment: 7. The water temperature is the: A. constant B. control C. dependent variable D. independent variable 8. The height of the launch is the: A. constant B. control C. dependent variable D. independent variable 9. Which of the following are constants in this experiment? A. Water temperature C. Both A and B B. Launch height D. None of the above 10.What type of graph would you use if you wanted to see if there are any trends in your data? (This question is NOT related to the chart above!) A.Bar C. Line B. Circle D. Scatter plot The diagram below shows milk being poured into a measuring cup. 11.Which property of the milk can be directly measured using the cup? A.Mass C. Solubility B. Density D. Volume 12. Which of the following methods would NOT be used for measuring volume? A. Measuring the volume of a marble using length x width x height B. Measuring the volume of a rubber stopper using the water displacement method C. Measuring the volume of Gatorade using a graduated cylinder D. Measuring the volume of a brick using length x width x height 13.When one variable increases, another variable increases. This is known as what kind of a relationship? A.Cumulative C. Inverse B. Direct D. Opposite 14.A car travels 50 meters in 25 seconds. What is the car’s average speed? A.2 m/s C. 75 m/s B. 0.5 m/s D. 25 m/s 15.What is defined as the rate of change of velocity over a period of time? A. Acceleration C. Speed B. Displacement D. Velocity 16.A car crashes into a tree. Passengers who are not wearing seatbelts continue to move in the direction the car was moving before it hit the tree, possibly being thrown from the car through one of its windows. This is an example of _______. A. Friction C. Inertia B. Gravity D. Newton’s 2nd Law of Motion 17. What is the net force acting on the box above? A. 10 N to the left C. 45 N to the right B. 30 N to the left D. 55 N to the right 18. The statement “An object at rest stays at rest and an object in motion stays in motion unless an outside force acts on it” is _______. A. Newton’s 1st Law of Motion C. Newton’s 2nd Law of Motion B. Newton’s 3rd Law of Motion D. Law of Conservation of Mass 19.The reason that a hot air balloon flies is because A.The hot air in the balloon is less dense than the air outside the balloon. B.The hot air in the balloon is denser than the air outside the balloon. C.The particles of hot air inside the balloon exert pressure on the balloon. D.The particles of cold air outside the balloon exert pressure on the balloon. 20. What is the difference between mass and weight? A. gravity C. direction B. friction D. they are the same 21. The greater the mass of an object: A. The easier it is for the object to start moving B. The greater its inertia C. The more balanced it is D. The more space it takes up 22. For the graph above, the independent variable is: A. speed C. acceleration B. force D. time 23. What is the acceleration of the ball at 2 seconds? A. 2 m/s C. 1 m/s 2 B. 2 m/s D. 1 m/s2 24. What does the line segment on the graph from 0 to 3 seconds represent? A. The speed remained constant B. The speed was increasing C. The speed was decreasing D. The speed was zero 25.How many simple machines are there? A. 6 B. 10 C. 12 D. 15 26.Machines are not very efficient because A.Work input is always less than work output. B.Energy is lost as heat due to friction. C.They multiply the input distance, not the input force. D.They require manpower or electricity to operate. 27.What type of energy does a bowling ball have as it travels down the lane toward the pins? A. Kinetic C. Mechanical B. Potential D. Thermal 28.The potential energy of an object is affected by A.The mass of the object. B.The height of the object above the ground. C.The speed of the object. D.Both A and B 29.Energy cannot be created nor destroyed; rather, it just changes forms. This statement is A.False B.The Law of Conservation of Energy C.The Theory of Energy Transformations D.The Kinetic Theory of Matter 30. Which of the following is NOT a method by which heat energy is transferred from a warmer object to a cooler one? A. radiation C. convection B. insulation D. conduction 31. All of the following are good conductors EXCEPT ________. A. silver C. aluminum B. copper D. air 32. A solid is a state of matter that has a(n) A. Indefinite volume and an indefinite shape B. Definite volume and a definite shape C. Definite volume and an indefinite shape D. Indefinite volume and a definite shape 33.Which graph best represents the relative distance between the particles of most substances in their solid, liquid, and gas states? A. B. C. D. 34. In which of the following situations would an object NOT float? A. The weight of the object is 20 N; the buoyant force is 20 N B. The object is less dense than water C. the object has a density of less than 1 g/mL D. The weight of the object is 20 N; the buoyant force is 10 N 35. Wires running between telephone poles are stretched tight in the winter but sag in the summer because: A. More birds land on the wires in the summer. B. The wires expand in the summer heat. C. People use more electricity in the summer. D. The wires partially melt in the summer heat. 36.When you open a bottle/can of soda, bubbles appear, rise to the surface, and burst out of the soda. This is an example of a A. Chemical change C. Physical change B. Chemical reaction D. Physical property 37. Which of these statements is false? A. Oxygen and copper are examples of elements B. Elements are pure substances. C. Atoms of different elements can combine to form compounds. D. Atoms of different elements are the same. 38. An uncovered pot of soup is simmering on a stove, and there are water droplets on the wall above the back of the stove. What sequence can you infer has occurred? A. Melting, then boiling C. Boiling, then condensation B. Freezing, then thawing D. Condensation, then boiling 39.The nucleus of an atom is made up of A.Electrons C. Protons B.Neutrons D. Both B and C 40. In which state of matter are most of the elements on the periodic table? A. solid C. liquid B. gas D. plasma 41. Which group of elements is least likely to bond with other elements? A. Alkaline earth metals C. Noble gases B. Halogens D. Nitrogen group 42.Elements in the same group have the same A. Number of neutrons B. Number of valence electrons C. Element classification (metal, nonmetal, metalloid) D. Atomic radii (are the same size) 43. Atoms will _______________ to become stable. A. Share electrons B. Transfer electrons C. Both A and B D. None of the above 44. How many electrons are needed in the outer energy levels of most atoms for the atom to be chemically stable? A. 1 C. 6 B. 4 D. 8 45. What property of aluminum allows it to be made into foil? A. Good conductor C. Malleable B. Ductile D. Solid at room temperature Use the equation below to answer questions 46 – 47 NiCl2(aq) + 2NaOH(aq) Ni(OH)2(s) + 2NaCl (aq) 46. NaOH is a ________ A. product B. reactant 47. This is an example of a balanced chemical equation. A. True B. False 48.How many atoms does (NH4)2S contain? A. 7 C. 10 B.8 D. 11 THANK YOU for taking this assessment. Please take your test question packet and answer sheet to the front lab desk and place them in the appropriate bins. Make sure your name is on your answer sheet. 8th Grade Physical Science Assessment Answer Sheet 1. ________ 25. ________ 2. ________ 26. ________ 3. ________ 27. ________ 4. ________ 28. ________ 5. ________ 29. ________ 6. ________ 30. ________ 7. ________ 31. ________ 8. ________ 32. ________ 9. ________ 33. ________ 10. ________ 34. ________ 11. ________ 35. ________ 12. ________ 36. ________ 13. ________ 37. ________ 14. ________ 38. ________ 15. ________ 39. ________ 16. ________ 40. ________ 17. ________ 41. ________ 18. ________ 42. ________ 19. ________ 43. ________ 20. ________ 44. ________ 21. ________ 45. ________ 22. ________ 46. ________ 23. ________ 47. ________ 24. ________ 48. ________