Stetson University
advertisement

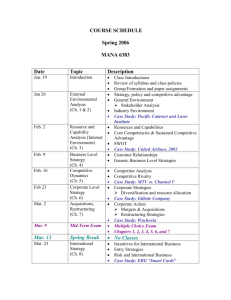
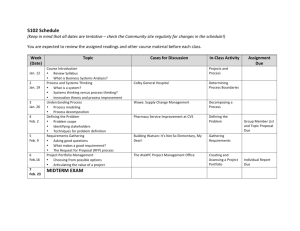
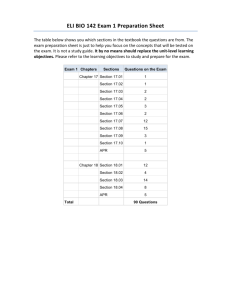
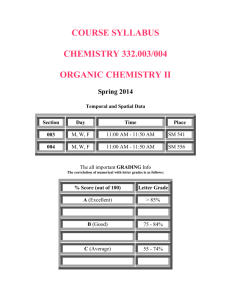
Stetson University Department of Chemistry Spring Semester, 2006 CY 102 - General Chemistry Instructor: Dr. Tandy Grubbs Office: 252, Sage Hall Office Phone: 822-8184 E-mail address: wgrubbs@stetson.edu Website: http://www.stetson.edu/~wgrubbs Office Hours: As posted on my door (or any time I am in my office and available) Text and Equipment: 1) “Chemistry: Molecules, Matter, and Change” by Atkins and Jones (W.H. Freeman, New York, 1999). 2) You should have access to the homework Solutions Manual; you may purchase your own copy from the bookstore or purchase and share a copy with a friend(s). 3) An accompanying Student Study Guide is available in the bookstore (not required). 4) “Laboratory Manual for Principles of General Chemistry” by J. A. Beran, 7th Edition, (Wiley, New York, 2004). 5) Non-programmable scientific calculator 6) Safety Goggles (available in the bookstore). Preparation: Lectures are designed to complement the material presented in the chapters. As such, it is best to read the sections in the text prior to coming to lecture. Exam questions will assess your mastery of chemical concepts from both the text and lecture. You will be notified if you are not responsible for certain subject matter in the text. As in most physical science courses, your ability to master the material will be greatly enhanced by completing a number of assigned homework problems (see page 3). While the Solution Manual will aid you when you encounter difficulties, you should practice enough problems so that you do not rely on it. If at any time you experience difficulties working assigned problems and/or comprehending material, you should seek my assistance. 1 Five-Minute Quiz: Every Wednesday, you will take a quick 2-4 question quiz at the beginning of class. The questions will be of two types: (1) conceptual in nature, involving quick response, recognitiontype, multiple choice, or fill-in-the-blank style questions, and (2) quantitative in nature, involving a straight-forward, one-step calculation to get to the answer. These quizzes will always be administered, unless a cancellation is previously announced or unless a major exam is scheduled for that day. The quiz will focus on the material presented within the last seven days. The lowest quiz grade will be dropped at the end of the term. No make-up quizzes will be scheduled, unless you miss one due to an official university function. Don’t be late and always remember to bring your calculator on quiz day. Attendance: It is in your best interest to attend all lectures. Roll will be taken periodically and a record of unexcused absences will result in partial (or full) loss of the 4% “class participation” portion of your final grade. Calculators and Exams: You will be allowed to use only non-programmable calculators during classroom examinations. Several inexpensive brands and models ($8.00 - $12.00) are available at local department and office supply stores (for example, the Casio FX-260 SOLAR or FX-250 HC and the Texas Instruments TI-25X or TI-30X are suitable models). Missed Lectures and Exams Policy: Any conflicts with scheduled lecture or exam dates due to your participation in an “official university function” should be discussed with me well in advance of the event. Make-up exams can be arranged if you miss an exam due to an illness. Tentative Lecture and Examination Schedule: Date Chapter and Topic Jan. 11,13,18,20 Liquids and Solids (Chapter 10) Jan. 23,25,27,30 Properties of Solutions (Chapter 12) Feb. 1 Exam #1 (Chapters 10 & 12) Feb. 3,6,8,10 Rates of Reaction - (Chapter 13) Feb. 13,15,17,20 Chemical Equilibrium (Chapters 14) 2 Feb. 22,24 Acids and Bases (Chapter 15) Feb. 27 Exam #2 (Chapter 13 & 14) Mar. 1,3 Cont. – Acids and Bases (Chapter 15) Mar. 6-10 Spring Break Mar. 13,15,17,20,22 Aqueous Equilibria (Chapter 16) Mar. 24 Exam #3 (Chapter 15 & 16) Mar. 27,29,31; Apr. 3 Direction of Chemical Change (Chapter 17) Apr. 5,7,10,12,17 Electrochemistry (Chapter 18) Apr. 19 Exam #4 (Chapters 17 & 18) Apr. 21,24,26 The Elements: Main Groups 1-8 (Lecture Notes) Apr. 24 Handout Take-Home Exam #5 (Lecture Notes) Final Exam Day Final Exam - Cumulative ACS Standardized Exam Suggested Homework Problems (from Atkins/Jones text): Chapter Problems 10 1,3,7,9,11,13,15,55,61,65,69,75,81,99 12 3,5,7,9,15,21,25,27,33,35,37,43,47,49,51,55,59,63,69,73,79,91 13 1,3,5,7,11,17,21,23,27,29,33,39,45,49,53,57,59,61,65,73,81 14 7,9,13,15,17,23,27,31,37,41,45,47,49,51,53,55,57,71,81,89 15 1,3,7,11,13,15,17,21,23,25,27,29,35,43,47,51,57,59,75,87 16 1,5,9,11,15,23,25,29,33,35,37,43,45,53,57,61,63,67,69,73,79,81,85 17 1,7,9,13,15,17,21,27,29,33,35,43,45,47,49,53,59,65,73,79 18 5,7,9,17,19,21,27,33,35,37,45,47,51,53,55,57,75,81,83,85,87 3 Course Evaluation: Graded Work Percentage of Total Exam 1 9 Exam 2 9 Exam 3 9 Exam 4 9 Take-Home Exam 5 5 Five Minute Quizzes 10 Final Exam 25 Laboratory 20 Attendance 4_______ TOTAL POINTS 100 Final Letter Grade Policy: 96.0-100 85.0-87.9 74.0-77.9 60.0-64.9 <50.0 A+ B+ C+ D+ F 92.0-95.9 81.0-84.9 69.0-73.9 55.0-59.9 A B C D 88.0-91.9 78.0-80.9 65.0-68.9 50.0-54.9 ABCD- Only For Teacher Education Majors (Chemistry 6-12): The following Florida Subject Area Competencies are covered in this class, pursuant to the Chemistry 6-12 Secondary Education degree. Knowledge of concepts of matter Knowledge of concepts of atomic theory Knowledge of concepts of periodicity Knowledge of concepts of chemical bonding Knowledge of chemical stoichiometry Knowledge of chemical kinetics and equilibrium Knowledge of acids and bases Knowledge of thermochemistry Knowledge of electrochemistry Knowledge of foundations Knowledge of laboratory skills and safety Familiarity with societal applications of chemistry 4