Ch. 1 Reading Notes
advertisement

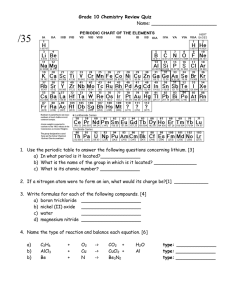
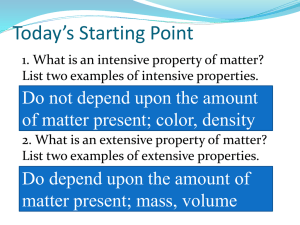
Modern Chemistry Chapter 1 Matter and Change Notes from Reading Section 2 Matter and Its Properties pages 10-18 Section 3 Elements page 20-24 1. Write the definition of matter and mass. 2. Write the definition of element and compound. 3. What are the smallest pieces of matter of a compound? An element? 4. What is the difference between extensive and intensive properties? Give examples of each. 5. Compare physical properties and changes. Give examples of each. 6. Write ideas about each state of matter in a table similar to the one below. Solid Liquid Gas Plasma 7. List four changes of state and the states of matter that are changed. 8. Compare chemical properties and changes. Give examples of each. 9. Describe the relationship between energy and changes. 10. Define the following terms: mixture, homogeneous mixtures, solutions, heterogeneous mixture, and pure substance. 11. Draw a concept map or flow chart to show the relationship between the following terms: matter, mixture, pure substance, homogeneous, heterogeneous, compound element. 12. Draw a diagram to show what information is given in a square of the periodic table. 13. Describe the name and characteristics of the columns of the periodic table. 14. Describe the name and characteristics of the rows of the periodic table. 15. Draw an outline of the periodic table and label the following regions: metals, nonmetals, metalloids and noble gases. 16. List characteristics of metal elements. 17. List characteristics of nonmetal elements. 18. List characteristics of metalloid elements. 19. List characteristics of noble gases.