Document
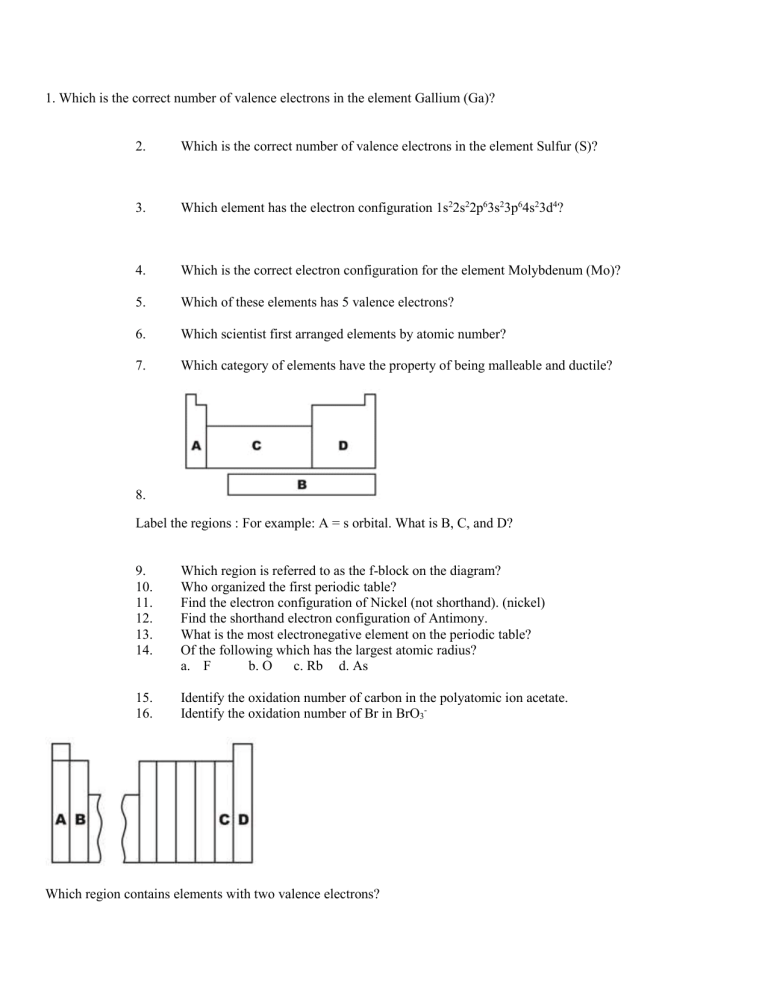
1. Which is the correct number of valence electrons in the element Gallium (Ga)?
2.
Which is the correct number of valence electrons in the element Sulfur (S)?
3.
Which element has the electron configuration 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 4 ?
4.
Which is the correct electron configuration for the element Molybdenum (Mo)?
5.
Which of these elements has 5 valence electrons?
6.
Which scientist first arranged elements by atomic number?
7.
Which category of elements have the property of being malleable and ductile?
8.
Label the regions : For example: A = s orbital. What is B, C, and D?
9.
Which region is referred to as the f-block on the diagram?
10.
Who organized the first periodic table?
11.
Find the electron configuration of Nickel (not shorthand). (nickel)
12.
Find the shorthand electron configuration of Antimony.
13.
What is the most electronegative element on the periodic table?
14.
Of the following which has the largest atomic radius? a.
F b. O c. Rb d. As
15.
Identify the oxidation number of carbon in the polyatomic ion acetate.
16.
Identify the oxidation number of Br in BrO
3
-
Which region contains elements with two valence electrons?
Which region contains elements with three valence electrons?
Which region contains the halogen family of elements?
Which region contains elements with an electron configuration that ends with p3?
Which trend follows for electronegativity?
Which trend follows for atomic radius?
Which trend follows for ionization energy?
Write the electron configuration for each letter on the above figure.
In which pair is the one on the left larger than the one on the right? a. Na, Rb c. Br, Br- b. S, Mg d. K, K+
Define ionization energy.
Identify the period and group numbers of the element with the electron configuration.
[Ne]3s 2 3p 4
According to (what person) periodic table, the physical and chemical properties of elements are periodic functions of their atomic weights.
Redox reactions involve the _____ of electrons.
Oxidation involves the _____ of electrons.
Indicate oxidation number of each element in the following:
Cyanide
Permanganate
Carbonate
Chromate dichromate
Indicate charges of the following:
Barium
Copper II
Aluminum silver