Introductory Learning Logs
advertisement

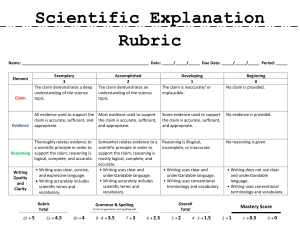
Unit 2 – Chemical Bonding Name: ________________________ Directions: Place a level of understanding, as aligned to the rubric, for each assessment that provides an opportunity to practice a given learning target. Make sure that you consider all aspects of the rubric before assigning a level on your own. Unit 2 – Chemical Bonding 1d Students know how to use the periodic table to determine the number of electrons available for bonding 2a Students know atoms combine to form molecules by sharing electrons to form covalent or metallic bonds or by exchanging electrons to form ionic bonds. 2b Students know chemical bonds between atoms in molecules such as H2, CH4, NH3, H2CCH2, N2, Cl2, and many large biological molecules are covalent. 2c Students know salt crystals, such as NaCl, are repeating patterns of positive and negative ions held together by electrostatic attraction. 2e Students know how to draw Lewis dot structures. 2g Students know how electronegativity and ionization energy relate to bond formation. Std Learning Target 2e 2a/2e 1c 2a/2g 2c 2a 2a/2b 2a/2e 2a/2b 2g LT 2.1 – I can draw and explain the significance of a Lewis Dot structure for any element on the Periodic Table. LT 2.2 – I can define and identify a cation and an anion. Additionally, I can predict the ion that will form for any element on the Periodic Table. LT 2.3 – I can define ionization energy and explain how it relates to the charge of the nucleus and the electron. Furthermore, I can explain how this trend changes as you move throughout the Periodic Table. LT 2.4 – I can hypothesize the compound that forms between two ions. Furthermore, I can discuss the role that electronegativity and ionization energy played in the formation of this ionic bond. LT 2.5 – I can explain how coulombic attractions lead to the formation of visible salt crystals. In this explanation, I can incorporate the idea of coulombic attraction, positive charge, and negative charge. LT 2.6 – I can define a metallic bond and explain the role of electrons in the formation of these bonds. LT 2.7 – I can identify a compound containing a covalent bond and discuss how electrons aid in the formation of covalent bonds. LT 2.8 – For a given covalent compound, I can draw a Lewis Dot diagram for the compound and explain how it illustrates the covalent bond. LT 2.9 – I can analyze the similarities and differences between metallic, covalent, and ionic bonds. LT 2.10 – Using the idea of electronegativity, I can predict the dipole moment of a given Lewis structure. LEARNING TARGET LOG Unit 2 – Chemical Bonding Name: ________________________ Marking/Grading Rubric (Levels) *The rubric below is for academic assessment. *Learning skills are scored separately and not included in an academic grade. Level 4: Knowledge and Reasoning: Consistently and accurately (90%) explain the connections between science skills, concepts, procedures, processes, and scientific reasoning by accurately using the language of science. Skill and Application: Consistently and accurately (90%) complete a variety of complex problems by connecting prior content, adapting knowledge, and justifying conclusions. Articulate the scientific concept using the most elegant method. Student Voice: Know what it is, how it works, why we care, connections Level 3: Knowledge and Reasoning: Explain the connections between most (80%) science skills, concepts, procedures, processes, and scientific reasoning by accurately using the language of science. Skill and Application: Accurately (80%) complete a variety of problems by connecting prior content and justifying conclusions. Articulate the scientific concept using the various methods from notes. Student Voice: Know what it is, how it works, sometimes why we care, some connections Level 2: Knowledge and Reasoning: Explain scientific skills, concepts, procedures, and processes by accurately (70%) using the language of mathematics. Skill and Application: Accurately (70%) complete expected problems by connecting prior content and attempting to justify conclusions. Demonstrate understanding of the scientific concept following a defined method from notes. Student Voice: Know what it is and how it works Level 1: Knowledge and Reasoning: Inconsistently use (60%) scientific skills, concepts, procedures, and processes by inconsistently (60%) using the language of mathematics. Skill and Application: Inconsistently (60%) complete some basic problems by using prior content and justifying conclusions. Partially demonstrate (60%) understanding of the scientific concept using one method. Student Voice: Know what it is and sometimes how it works No credit Student evidence of knowledge, reasoning, product, and performance is below 60%. Student receives descriptive feedback and intervention support and must redo before scoring. Not learning is not an option. Student Voice: Do not know what it is and how it works Identify your level:_______ Justify your mark based on your self-assessment, evaluating your knowledge, reasoning, performance skill, and product: Teacher’s identification of your level: _________ Further Justification: LEARNING TARGET LOG