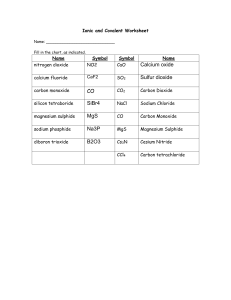
08-Nomenclature multiple ionic charges teacher
advertisement

` SNC2D - Chemistry Ionic Compounds with Multivalent Metals Most transition metals and some metals in group 14 (or IVA) are multivalent (they have more than one possible valence). They can form different numbers of bonds depending on the conditions. There are 2 systems of rules for naming compounds with metals of variable valence: IUPAC (international) and “Classical” (“ous-ic”). We will learn the IUPAC system. International System Writing Formulas: 1. Use the regular methods for writing formulas of ionic compounds (ionic charge or criss-cross). The metal is written first, and then the non-metal. 2. For the metal, use the charge given in brackets. Examples: copper (II) nitride tin (IV) oxide Cu+2 N-3 Sn+4 Cu3N2 Sn2O4 O-2 Reduces to SnO2 Writing Names: 1. From the formula, determine the total amount of negative charge. 2. Multiply the anion subscript by the charge on the anion to determine the negative charge. 3. The amount of positive charge must equal the amount of negative charge. Determine the charge on the cation by dividing the total positive charge by the number of cations (from the subscript in the formula). 4. Write the names of the elements in the same order as the formula (metal first, followed by the non-metal with “ide” suffix) 5. Use Roman numerals in brackets following the metal to indicate the charge of the cation. Examples: a) 1 FeI2 ` SNC2D - Chemistry b) Ni2O3 c) Cu3N2 Therefore the name is copper (II) nitride Practice I. Write the correct formulas for the following: 1. cobalt (II) sulfide 11. tin (IV) iodide 2. chromium (III) bromide 12. copper (I) oxide 3. lead (II) oxide 13. lead (III) phosphide 4. gold (I) oxide 14. manganese (IV) oxide 5. tin (II) sulfide 15. nickel (III) sulfide 6. iron (III) nitride 16. lead (IV) bromide 7. bismuth (V) carbide 17. scandium (II) iodide 8. gold (I) sulfide 9. tin (IV) carbide 10. copper (II) nitride Practice II. Write names for the following 3. PbO2 1. SnF4 4. HgCl 2. FeS 5. Ni2O3 2 ` 6. Cu3N 7. AuF3 8. CuI 9. PbCl4 10. CuCl2 11. CoF3 12. FeO 13. MnCl7 14. Cr3N2 15. Sn3P2 3 SNC2D - Chemistry