results - Tufts University
advertisement
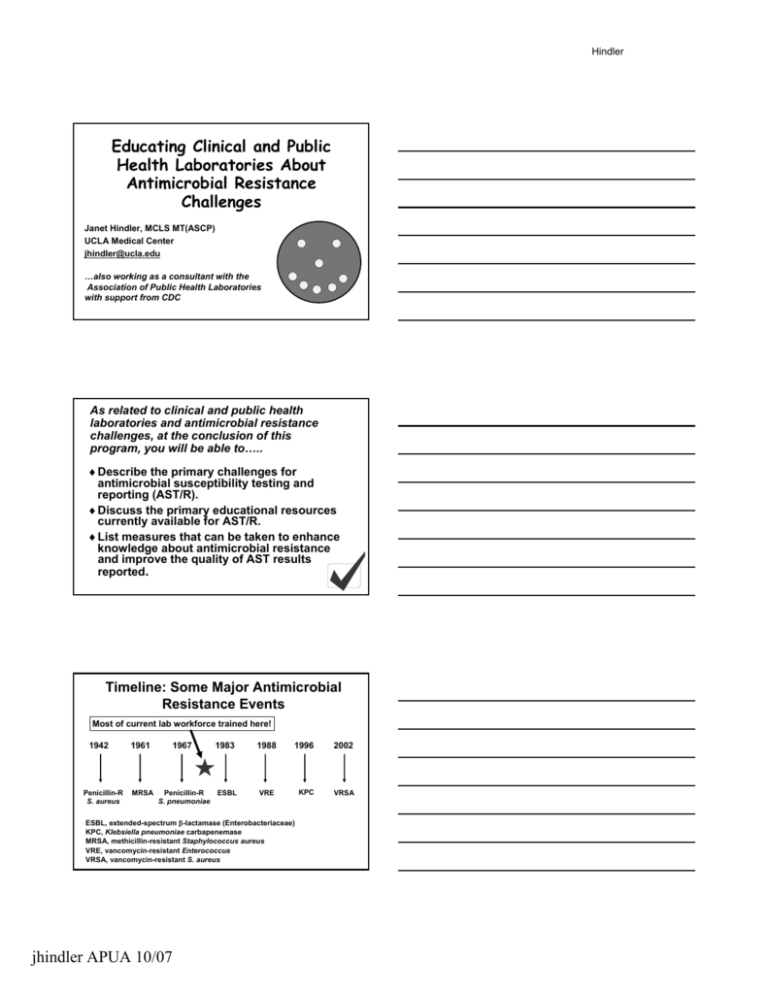
Hindler Educating Clinical and Public Health Laboratories About Antimicrobial Resistance Challenges Janet Hindler, MCLS MT(ASCP) UCLA Medical Center jhindler@ucla.edu …also working as a consultant with the Association of Public Health Laboratories with support from CDC As related to clinical and public health laboratories and antimicrobial resistance challenges, at the conclusion of this program, you will be able to… to….. ♦ Describe the primary challenges for antimicrobial susceptibility testing and reporting (AST/R). ♦ Discuss the primary educational resources currently available for AST/R. ♦ List measures that can be taken to enhance knowledge about antimicrobial resistance and improve the quality of AST results reported. Timeline: Some Major Antimicrobial Resistance Events Most of current lab workforce trained here! 1942 Penicillin-R S. aureus 1961 MRSA 1967 1983 Penicillin-R ESBL S. pneumoniae 1988 1996 2002 KPC VRSA VRE ESBL, extendedextended-spectrum β-lactamase (Enterobacteriaceae) KPC, Klebsiella pneumoniae carbapenemase MRSA, methicillinmethicillin-resistant Staphylococcus aureus VRE, vancomycinvancomycin-resistant Enterococcus VRSA, vancomycinvancomycin-resistant S. aureus jhindler APUA 10/07 Hindler What is required of USA labs and what information is available for AST/R? Please note: AST/R = antimicrobial susceptibility testing and reporting “Specific” Specific” mandates by accrediting / certifying agencies for clinical and public health laboratories regarding AST/R… AST/R…. It is mandatory for laboratory to: As related to AST/R… AST/R…. Document competency of staff Does not specifically address AST/R Include organisms with specific resistance mechanisms (3 - 8/year) Participate in proficiency testing surveys Perform adequate quality control Very specific requirements for AST/R Perform appropriate testing / reporting of patient specimens Very few specific requirements for AST/R In terms of information for effective AST/R, what is readily available to clinical and public health laboratories? Information readily available for: Selecting organisms to test + Selecting drugs to test +++ Testing methods ++++ Reporting results ++ +, little information available; ++++, much information available available jhindler APUA 10/07 Hindler Sources of Information for AST/R ♦Text books / manuals – CLSI (Clinical and Laboratory Standards Institute) documents (primary resource) http://www.clsi.org/ – Isenberg, HI. (ed). 2004. Clinical Microbiology Procedures Handbook, Handbook, 2nd edition. Sxn. Sxn. 5. ASM Press, Washington, DC. – Murray, PR, EJ Baron, JH Jorgensen, ML Landry and MA Pfaller (eds). 2007. Manual of Clinical Microbiology, Microbiology, 9th ed. pp. 10751075-1269. ASM Press, Washington, DC. Sources of Information for AST/R (con’ (con’t) ♦Continuing education programs sponsored by: – Professional organizations – Commercial companies – Public health organizations ♦Various websites, listserves Primary Challenges for AST/R jhindler APUA 10/07 Hindler What are the primary challenges for AST/R in clinical and public health laboratories? ♦ Performing susceptibility tests when appropriate (limit over or under reporting) ♦ Getting access to the most recent CLSI AST/R recommendations and keeping up with the recommendations ♦ Applying all of the “special” special” testing/reporting rules (need artificial intelligence!) What are the primary challenges for AST/R in clinical and public health laboratories? (con’ con’t) ♦ Dealing with conflicting recommendations (e.g., CLSI vs. FDA breakpoints) ♦ Having confidence in performance of commercial testing systems ♦ Deciding how far a smaller lab should go with AST/R ♦ Defining the role of public health laboratories ♦ Communicating results effectively Specimen: Leg wound Diagnosis: Hypertension GS: Many GPC clusters Many pleomorphic GPR No WBCs Should AST be performed? Culture: Many coagcoag-neg staphylococcus Many diphtheroids Few E. colicoli-like colonies Few ProteusProteus-like colonies jhindler APUA 10/07 Hindler CLSI (Clinical and Laboratory Standards Institute, formerly NCCLS) Documents CLSI ♦ M2M2-A9 Disk diffusion (2006) Documents ♦ M7M7-A7 MIC (2006) ♦ *M100*M100-S17 Tables with breakpoints, QC ranges, drugs to test (2007) ♦ M11M11-A7 Anaerobes (2007) ♦ M39M39-A2 Cumulative antibiograms (2005) ♦ 4545-A Infrequently Isolated / Fastidious Bacteria (2006) *updated yearly; others updated every 33-5 years Staphylococcus aureus AST/R Rules Agent Cefazolin Clindamycin Daptomycin Erythromycin Linezolid Rule Report “R” for MRSA even if test result is ”S” Perform D zone test for inducible resistance if erythromycinerythromycin-R and clindamycinclindamycin-S Do not report on urine isolates Only “S” breakpoint; send isolate to reference lab if not “S” Erythromycin results predict those for azithromycin and clarithromycin Do not report on urine isolates Only “S” breakpoint; send isolate to reference lab if not “S” Staphylococcus aureus AST/R Rules (con’ (con’t) Agent Oxacillin Penicillin AST/R Rules AST/R Rules Rule Cefoxitin detects mecA mecA-mediated resistance better than oxacillin Perform β-lactamase test if MIC ≤0.12 µg/ml Rifampin Add report note “rifampin should not be used alone for antimicrobial therapy” therapy” Tetracycline If S, consider doxycycline and minocycline “S”; if R, doxycycline and/or minocycline may be “S” Trimeth-sulfa Ignore 20% growth when determining endpoint Trimeth--sulfa Vancomycin Verify any result with MIC >2 µg/ml jhindler APUA 10/07 Hindler How can we ensure AST/R rules and recommendations are followed consistently? AST/R Rules Need “Artificial Intelligence” Intelligence” ♦ Flag organisms needing further testing ♦ Report appropriate drugs ♦ Edit “S” or “I” results to “R” ♦ Flag atypical / inconsistent results AST/Report Rules ♦ Add comments to report Examples of FDA vs. CLSI Breakpoints S, I, R Breakpoints (µ (µg/ml) Drug / bug Oxacillin Staphylococcus FDA CLSI ≤2, -, ≥4 ≤2, -, ≥4 none ≤2, 4, ≥8 ≤2, 4, ≥8 none ≤4, 88-16, ≥32 ≤2, 44-8, ≥16 Colistin P. aeruginosa Tigecycline Enterobacteriaceae Vancomycin S. aureus Conflicting recommendations Currently, diagnostic manufacturers must use FDA breakpoints Clinical laboratories can use CLSI or FDA breakpoints Need “harmonization” harmonization” of breakpoints How well does an “I” or “R” result predict KPCs? KPCs? (N = 31 KPC producers; 45 nonnon-KPC producers) Sensitivity/specificity (%) Reliability of methods Carbapenem “I” or “R” result Method Meropenem Imipenem Ertapenem Reference BMD 94/98 94/93 97/89 Etest 58/96 55/96 90/84 Disk diffusion 71/96 42/96 97/87 Vitek Legacy 52/98 55/96 NA Vitek 2 48/96 71/96 94/93 MicroScan 84/98 74/96 100/89 Phoenix 61/98 81/96 NA Sensititre 42/98 29/96 NA NA, not applicable (range not low enough or drug not available on on panel) Anderson et al. 2007. J Clin Microbiol. 45:2723. jhindler APUA 10/07 Hindler Example: types of labs doing AST/R = lab in 5050-75 bed rural hospital = lab in >500 bed tertiary care center or reference lab What testing menu might the small rural hospital laboratory adopt for AST/R? ♦ S. aureus – Must all AST/R rules be considered? ♦ E. coli / Klebsiella spp. – Should ESBL testing be performed? – Should ertapenem be tested to detect KPC (Klebsiella (Klebsiella pneumoniae carbapenemase) producers? ♦ Should other bacteria be tested? ♦ Should all AST be sent out? Smaller labs – Turn around time considerations and AST/R?? Issues for the small rural hospital laboratory re: AST/R ♦ How can small lab have a limited AST/R menu when any type of resistant organism is possible in any patient? ♦ How can limited staff be adequately trained to perform all AST/R reliably? – Staff often do microbiology “part time” time” Smaller labs and AST/R?? jhindler APUA 10/07 Hindler Specimen: Peritoneal fluid Diagnosis: Appendicitis Pseudomonas aeruginosa MIC (µ (µg/ml) ceftazidime 8S Communicate ciprofloxacin 1S results effectively gentamicin 2S piperacillin 16 S “Combination therapy (e.g. β-lactam + aminoglycoside or β-lactam + fluoroquinolone) should be considered for serious P. aeruginosa infections” Cumulative Antibiogram 2006 Communicate results effectively N Am Cfaz Cftrx Cip Gm TT-S 61 92 95 92 97 76 E. cloacae 144 - - 71 95 88 84 221 - - 10 76 88 - E. coli P. aerug 729 % Susceptible %S data compiled according to CLSI M39M39-A2. Public Health Labs and AST/R jhindler APUA 10/07 Hindler How are public health laboratories involved with AST/R? ♦ Most do no AST and have limited expertise in AST ♦ If testing is done: – – – – – Campylobacter jejuni / coli Neisseria gonorrhoeae Salmonella spp. Streptococcus pneumoniae Staphylococcus aureus ♦ Method primarily disk diffusion and/or Etest How are public health laboratories involved with AST/R? (con’ (con’t) ♦ Some state PH labs are helping to educate clinical laboratories with AST/R; examples: – New Jersey – cumulative antibiogram project – New York – proficiency testing program – Washington – regional technical workshops How are public health laboratories involved with AST/R? (con (con’’t) ♦ Some state PH labs have affiliation with clinical lab (university) and can provide more support for clinical laboratories; examples: – Nebraska – clinical lab is resource for problem isolates – Iowa – collect, test, provide feedback on specific isolates ♦ Some state PH labs do limited surveillance for antimicrobial resistance jhindler APUA 10/07 Hindler Expectations….. PH Lab expects Clin Lab to provide: Clin Lab expects PH Lab to provide: • Isolates w/ unusual “R” (e.g., VRSA) • Verification of unusual results (or sends to CDC) • Technical information from CDC / Others • Notice of “R” events (e.g., VRSA) • Notice of training events ….maybe • Cumulative antibiogram data …maybe • Regional antibiogram data • CLSI AST standards What 10 steps can we take to educate clinical and public health laboratories about antimicrobial resistance challenges and improve practices to optimize reporting of “quality” quality” results? What can we do? 1. Provide the latest CLSI standards to all labs that perform AST/R for patient care. 2. Continue to develop/promote/present educational programs to help labs “interpret” interpret” and apply the latest CLSI standards (routine AST/R and cumulative antibiograms). 3. Develop “standards of practice” practice” a) When to perform AST (which organisms to test from various specimen types) b) How to most effectively report results c) Enhanced guidelines for interpreting results jhindler APUA 10/07 Hindler What else can we do? 4. Investigate ways a small rural lab could best handle AST/R. 5. Continue to pursue harmonization of FDA and CLSI breakpoints. 6. Encourage diagnostic manufacturers to reevaluate their systems regularly to determine the system’ system’s abilities to detect resistance in “contemporary” contemporary” pathogens. What else can we do? 7. Encourage diagnostic manufacturers and software vendors to improve artificial intelligence for AST/R. 8. Develop/promote/present educational programs to assist PH labs to better serve as a resource (or identify an alternative resource) for AST/R in their community. 9. Interact with accrediting and regulatory agencies to help their surveyors identify inappropriate AST practices that might lead to medical errors. What else can we do? 10. Provide validated materials (e.g., ppt. ppt. presentations and other) that can be used by others to emphasize effective ways to confront emerging resistance; audiences: ¾ Clinical lab ¾ Public health (lab/epi (lab/epi)) ¾ MDs ¾ Infection Control ¾ Pharmacy jhindler APUA 10/07 Hindler Counts et al. 2007. JCM. 45:2230. Reports of recent successes Rosner et al. 2007. Clin Lab Sci. 20:146. Might there be mechanisms to further educate clinical and public health laboratories about antimicrobial resistance challenges?? http://www.idsociety.org/STAARAct.htm http://www.idsociety.org/STAARAct.htm jhindler APUA 10/07