34 writing formulae for ionic compounds(ii)
advertisement
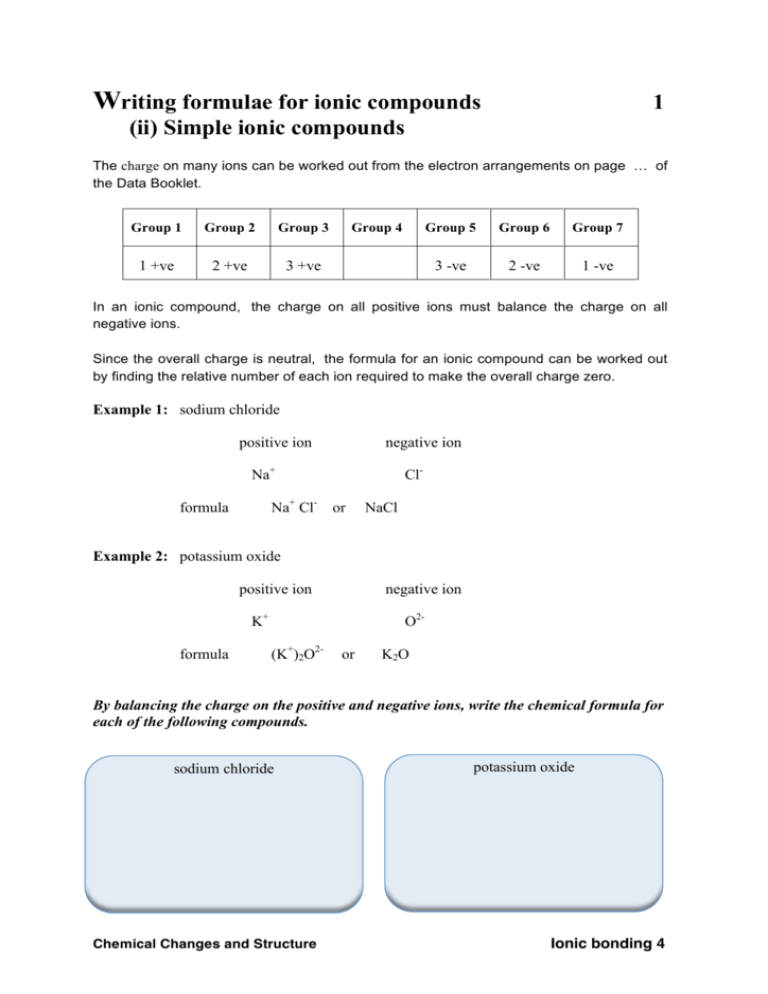
Writing formulae for ionic compounds 1 (ii) Simple ionic compounds The charge on many ions can be worked out from the electron arrangements on page the Data Booklet. Group 1 Group 2 Group 3 1 +ve 2 +ve 3 +ve Group 4 Group 5 Group 6 Group 7 3 -ve 2 -ve 1 -ve of In an ionic compound, the charge on all positive ions must balance the charge on all negative ions. Since the overall charge is neutral, the formula for an ionic compound can be worked out by finding the relative number of each ion required to make the overall charge zero. Example 1: sodium chloride positive ion negative ion Na+ Na+ Cl- formula Clor NaCl Example 2: potassium oxide positive ion negative ion K+ formula O2(K+)2O2- or K2 O By balancing the charge on the positive and negative ions, write the chemical formula for each of the following compounds. sodium chloride Chemical Changes and Structure potassium oxide Ionic bonding 4 Writing formulae for ionic compounds 2 (ii) Simple ionic compounds calcium sulphide radium oxide lithium iodide magnesium bromide rubidium oxide strontium nitride Chemical Changes and Structure Ionic bonding 4