Densities of Common Substances Worksheet
advertisement

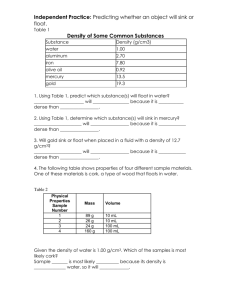
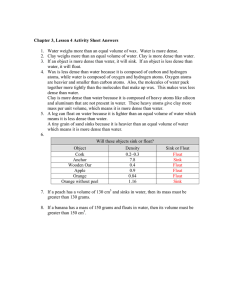
Name ____________________________________ Period_________ Densities of Common Substances Substance Water Ice Gasoline Lead Cork Iron Density 1.00 g/mL 0.92 g/mL 0.70 g/mL 11.30 g/mL 0.24 g/mL 7.90 g/mL Substance Seawater Aluminum Oil Gold Rubber Bone Density 1.025 g/mL 2.70 g/mL 0.90 g/mL 19.3 g/mL 1.10 g/mL 2.45 g/mL Use the chart above to answer the following questions. SHOW YOUR WORK! 1. Identify the substance that has a mass of 70 g and a volume of 100 mL. Will this substance float or sink in oil? 2. This substance has a volume of 200 mL and a mass of 180 g. What is it? Will this substance float or sink in water? 3. This substance has a mass of 82 g and a volume of 30.3 mL. What is it? Will this substance float or sink in water? 4. Identify the substance that has a mass of 4.41 g and a volume of 1.8 mL. Will this substance float or sink in water? 5. This substance has a volume of 77 mL and a mass of 85 grams. What is it? Will this substance float or sink in oil? 1. Write the formula for density. Density = 2. Complete the chart below. Object Volume (cm3 ) Mass (grams) A 3 9 B 2 12 C 3 15 D 25 50 Density (g/ cm3) 3. Which of the above objects has the greatest density? 4. Which of the above objects has the least density? C Use the picture on the right to answer questions 5 -9. 5. The bead was placed into a test tube as shown on the right. Is the bead more dense or less dense than substance C? B 6. What is the volume of the bead if the starting volume of the liquids was 25 mL? 7. What is the density of the bead if its mass is 8 grams? 8. Predict the density of substance B if substance A has a density of 4 g/cm3? 9. Looking at the graduated cylinder, list the letters in order least dense to most dense. A