Review for the midterm, some solution chemistry and some acids
advertisement

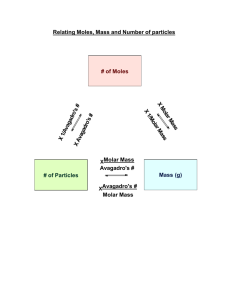
Review for the midterm, some solution chemistry and some acids and bases This packet belongs to: # Assignment Assigned Due 1 Read part of section 4.3, pg 121-124 2 Read section 4.5. Look over all the sample exercises in the section and do the practice exercises. 3 Read Section 4.6. Look at sample exercises 4.16 and 4.17 and do the corresponding practice exercises. 4 Pg 146 #21-25 (goes with assignment 1) 5 Packet page 1-2 (midterm rev) 6 Packet pg 3,4 (chemistry rev) 7 Packet pg 5,6 (More review!) 8 Packet pg 7,8 (solutions) 9 Packet 9,10 (acids/bases/pH) Schedule 1/3: Lecture on atom packing and the Zn chip lab. Lab time/class time for labs. 1/7: Solutions lecture and problems. Some midterm review. Labs are due. 1/9: Solutions and acids and bases lecture. Some midterm review. 1/11: Acids/bases/pH, some midterm review. Maybe some demos. 1/14: 7 period day. Midterm Review. Review for the Midterm The midterm is challenging. Knowers will finish quickly, forgetters will take a long time. The test is about 50 questions, mostly multiple choice and true/false. There will be a handful of problems that you will have to do out and show work. There are no curveballs. Study old tests. DO NOT just look them over. Cover up your old answers and redo the questions. There will be a review session after school if people desire one. We will schedule that soon. A. 1. Matter; Basic Concepts a. List those properties which differentiate between matter which is pure and that which is not. b. What is meant by the term "homogeneous mixture"? Name one. 2. What are the different types of pure matter? 3. a. b. List 5 physical properties of water. List 5 chemical properties of water. B. 1. Mathematics in Chemistry What is density? 2. Why are significant figures important? 3. A quantity of gold is stolen from Fort Knox. The only possible witness is a guard who claims to have seen a thief running with a bucket of gold in one hand from the main vault. The bucket was described to be about a 2 gallon bucket when full, and the density of gold is 19.3 g/cm3. Comment on the validity of the statement made by the guard. 4. A fathom, used to measure water depths, is defined as exactly 1.8288 m. What is the depth in fathoms of Lake Superior, which is 1302 feet deep at its maximum depth? 5. A car weighing 4.85 X 103 lbs has a mass of how many kilograms? C. 1. Nomenclature; Formula-writing Write the formula or names. a. Ferric phosphate b. PCl3 2. b. d. Write the old and the new names for these. a. SnSO4 b. plumbous nitrite N2O5 Cu2O D. 1. Reactions; Equations a. Reactive metal + acid c. Acid + carbonate b. Carbonate heated 2. a. N2O5 + H2O HNO3 b. H2SO4 + KOH E. 1. Stoichiometry Li3N reacts with water to produce ammonia and lithium hydroxide. What is the mass of ammonia produced when 5.38 g of lithium nitride reacts? F. Percent Composition/The Mole 1. 23.6 g of Al is how many moles? 2. How much does 3.25 moles of silicon dioxide weigh? 3. How many molecules of silicon dioxide are in the previous problem? 4. Consider aluminum oxide. If there are 0.0231 moles of it: a. How many grams of aluminum oxide are there? b. How many moles and how many molecules of aluminum are there? c. How many moles and how many molecules of oxygen are there? d. What is the mass percent of oxygen? 5. A compound is 32.79% sodium, 13.02% Al, and 54.19% fluorine. What is the compound’s empirical formula? G. 1. Lab Questions Describe how to produce and collect two silver compounds that are insoluble in water. 2. Describe how to produce, collect, and test hydrogen gas. In addition, here are questions in the book which are applicable. Feel free to work on others at the end of these chapters to prepare yourself. Math in Chem Chapter 1 pg 29: 1, 13, 15, 27, 47 Naming chemicals Chapter 2 pg 68: 29, 53, 55, 57, 49, 61, 59, 91, 90, 87 Stoichiometry, balancing, percent composition Chapter 3 pg 105: 5, 7, 41, 39, 27, 55, 61, 59, 75, 73 Reactions/equations The book covers reactions and equations differently than we did in class. Be sure to go over old worksheets, notes and tests on this unit. Also, review old tests, quizzes and worksheets!! Chemistry Review 10. A student performs an experiment to determine the freezing point of water. Three values are obtained: -2.0°C, -2.2°C, and -2.1°C. The thermometer has a mark for each degree. Which of the following statements is true? These value a) are precise but not accurate. b) are neither accurate nor precise. c) are accurate and precise. d) are accurate but not precise. e) should not have a tenths place. 1. Which number is incorrectly converted to standard scientific notation? a) 842.6 x 104 = 8.426 x 106 b) 0.00456 x 10-8 = 4.56 x 10-11 c) 0.00452 x 106 = 4.52 x 109 d) 454 x 10-8 = 4.54 x 10-6 2. A certain liquid has a density of 2.67 g/cm3. What volume in liters would 1340 g of this liquid occupy? 3. One side of a cube measures 1.55 m. What is the volume of the cube in ml? 4. Which name is correct? a) MgS, magnesium sulfite b) Li2O, dilithium monoxide c) FeCl3, iron(III) chloride d) Ca3N2, calcium nitrite e) HBr, bromic acid 12. How many atoms of nitrogen are in 10. g of NH4NO3? c) 3.0 x 1023 a) 1.5 x 1023 b) 2 d) 7.5 x 1022 5. When the following equation is balanced, the coefficient of H2S is FeCl3 + H2S Fe2S3 + HCl 6. The combustion of propane is given below. It is unbalanced. 2.5 moles of O2 produces how many moles of water? C3H8 (g) + O2 (g) H2O (g) + CO2 (g) 7. Octane burns according to the following equation: 2C8H18 + 25O2 16CO2 + 18H2O How many grams of CO2 are produced when 5.00 g of C8H18 are burned? 8. 9. 11. Convert the following description into a balanced equation: When ammonia gas, NH3(g), is passed over hot sodium, hydrogen gas is released and sodium amide, NaNH2, is formed as a solid product. Which of the following is named incorrectly? a) BCl3--boron trichloride b) PCl5--phosphorus hexachloride c) CS2--carbon disulfide d) IF7--iodine heptafluoride e) SiO2--silicon dioxide A spherical ball of lead has a diameter of 5.0 cm. What is the mass in grams of the sphere if lead has a density of 11.34 g/cm3? (The volume of a sphere is V = (4/3) r3.) 13. What is the percent yield of Ca(OH)2 in the reaction CaO + H2O → Ca(OH)2 if 5.00 g of CaO is reacted with excess water and 6.11 g of Ca(OH)2 is collected.? 14. What is the empirical formula of a compound that contains 27.0% sulfur, 13.5% O and 59.5% Cl by mass? 15. If your partner measured the mass of a rock as 15.5 g and you measured the volume as 7.432 cm3, how should the density be reported? 16. All of the following are homogeneous mixtures, except a) Brass b) 3 M Copper(II) sulfate solution c) ethanol 17. Which of the following is the correct formula for an acid? a) HS c) HNO2 b) H2SO2 d) H2CO2 18. What is the difference between an endothermic and an exothermic reaction? 19. List the diatomic elements. 20. What does a catalyst do? 21. Change to scientific notation and indicate the number of sig figs for each. a. 2.0100 g b. 2.30 cm c. 23,000 miles d. 0.0000231 m ____________ __________ ______________ _______________ 22. Write the names of the following compounds. a. Mg(OH)2*8H2O b. Al2(Cr2O7)3 c. H2SO3 d. CuCO3 e. Cu2SO4 f. AgBr g. N2O5 h. NH4ClO 23. Write the formulas for the following compounds. a. stannous fluoride b. xenon dioxide c. copper (II) carbonate d. sodium chlorite e. bromous acid f. dinitrogen monoxide 24. Given the following reagents, write completed balanced equations that can do the following. For each reaction, indicate the reaction type. (3 each equation) Fe2O3 Al(OH)3 H2SO4 Al CuO NH4Br HCl CaCO3 a. Make aluminum oxide. b. Make water 4 ways. c. Make hydrogen gas. 25. AlN + H2SO4 Al2(SO4)3 + NH3 If 34.5 g sulfuric acid is reacted with excess aluminum nitride given the unbalanced equation above, how many grams of ammonia (NH3) will form? If 1 mole of gas = 24.8 L, how many liters of ammonia will form? 26. 3.45 g aluminum hydroxide reacts with 2.54 g of sulfuric acid in a double displacement reaction. What mass of the salt is formed? What is the limiting reagent, what is in excess, and how much of the excess reagent remains? More Chemistry Practice Midterm Questions! 1. Name the following: CuSO4 NaOH HBr CCl4 2. How many significant figures do the following numbers have? Convert each to scientific notation. Sig figs Sci. Notation 30.21 103.0 0.00378921 3.00000 3. I can run 18 mph. How fast is this in m/s? How much time would it take to run 400 meters? 4. Give an example of five pure substances. 5. What is the difference between a homogeneous and a heterogeneous mixture? 6. 378.56 g of cobalt (II) chloride is how many moles? 7. A solution of sodium hydroxide is made by dissolving 82.78 g of sodium hydroxide into 2 liters of water. How many moles of sodium hydroxide are dissolved in the water? 8. What is the mass percent composition of each element in sulfurous acid? 9. What is produced when a solution of magnesium sulfate is mixed with sodium hydroxide? 10. How much metal is produced if 2.67 g of zinc are reacted in excess copper (II) sulfate? 11. Draw a fully labeled energy diagram for the decomposition of hydrogen peroxide. 12. AgNO3 + NH4Cl + Cu Mg(OH)2 _________ + _________ + __________ Type: Type: Problems with solutions You may recall that on one of the first days of the year in this class, a "solution" was defined as a "homogeneous mixture". In an aqueous solution, water is the "solvent", and whatever is dissolved in it is the "solute". (Solvent + solute = solution.) When ionic substances (such as magnesium chloride) dissolve in water, it is the ions that are dissolved -- in other words, the MgCl2 (s) breaks apart into Mg+2 ions and Cl1 ions. Of course, in this case, there would be twice as many chloride ions as magnesium ions in solutiion. A solution is a mixture because it may consist of any ratio of solute to solvent. There are many ways to express the concentration of a solution: parts per million (ppm), percent (%), "formality", "normality", and "molarity". The big "M" that you've often seen after a number on a flask in a lab stands for "molarity", and is a common unit used for a concentration of a solution. "Molarity" is defined as "moles of solute divided by liters of solution": [Molarity ] = moles solute liters solution The square brackets around a symbol are used to indicate concentration in molarity. You may be asked (in lab or on paper) to construct a particular amount of a solution, with a specific concentration. Solutions are either constructed by measuring a number of moles of a solid, then dissolving it in the solvent until the volume is reached, or by diluting a (more concentrated) "stock" solution with pure solvent. If you are asked to dilute a stock solution, use this formula: MsolVsol = MstockVstock , where M stands for "molarity", V stands for "volume", "sol" stands for the "solution" you are making, and "stock" refers to the stock solution. If, on the other hand, you are asked to make a solution starting with solid material to dissolve, you'll need to use dimensional analysis. For example, if you were asked to make 147 ml of a 2.5 M NaCl solution (starting with solid NaCl), do this: 1liter 2.5moles NaCl 1liter 1000ml (?)gNaCl = 147ml 58.5 g NaCl 1mole NaCl = 21.5 g 1. Bart is given a solution and is told that it is a 6.50 M MgSO4 solution. Bart carefully measures 100.0 ml of the solution in a volumetric flask, and pours it into a previously-weighed evaporating dish. He then slowly heats the solution and drives off the water. What should the mass of the solid magnesium sulfate be which is left in the dish? 2. Another student, Barb, is handed a solution of MgSO4 and is asked to determine the concentration. She carefully measures 25.0 ml using a pipette, and heats the solution in an evaporating dish. After the water is evaporated, there is 12.50 g of solid remaining in the dish. What is the molarity of the solution? 3. How could you make 200 ml of a 1.5 M HCl solution from a stock 12 M HCl solution? 4. How many grams of zinc sulfate are in 25.0 ml of a 0.355 M solution? 5. Write the ionization reaction which occurs when each of the following compounds are placed in water. a. NH4Cl b. NaOH c. Al2(SO4)3 d. H2O 6. Three students make up different solutions of copper(II) nitrate: Alfred dissolves 13.75 g in 100 ml; Betty dissolves 25.35 g in 330 ml; and Cornelius dissolves 10.02 g in 86.0 ml. Who made the strongest, and who made the weakest solution? 7. 0.100 liters of a 2.5 M NaOH solution is diluted to 0.350 liters (by adding 250 ml of distilled water to it.) What is the concentration of the new solution? 8. a. What is the concentration of H+ ions in a 2.0 M HCl solution? b. What is the concentration of Mg+2 ions in a 0.30 M Mg3(PO4)2 solution? c. How many grams of chloride ions are in 200 ml of a 1.5 M CuCl2 solution? pH Problems 1) What is the molarity of a solution that has 450 grams of sodium chloride in 800. mL of water? 2) What is the molarity of a solution that contains 100. grams of iron (II) nitrate in 2.4 liters of water? 3) What is the pH of a solution that contains 2.4 x 10-5 moles of hydrobromic acid in 0.50 L of water? 4) What is the pH of a solution that contains 25 moles of nitric acid dissolved in 5000. liters of water? 5) What is the pH of a solution that contains 0.0090 grams of hydrochloric acid in 100. mL of water? 6) What is an acid/base indicator used for? 7) Define “titration”: 8) In a few steps, describe how you would titrate a base of unknown concentration with an acid with concentration 1.0 M. 9) I did a titration where it took 50. mL of 0.10 M hydrochloric acid to neutralize 500. mL of a base with unknown concentration. Using this titration information, what was the concentration of the base? 10) I did a titration where it took 25 mL of 5.0 M NaOH to neutralize 1.0 L of an acid with unknown concentration. Using this information, what was the concentration of the acid? 11) What is the difference between a “strong” acid or base and a “weak” acid or base? (Look in the book – this does not really refer to concentration.) 12) What is the value of the “ion-product constant” (Kw) of water? 13) What is the meaning of the “p” in “pH”? 14) Prove mathematically (by showing everything!) that the pH of pure water is 7. 15) What is the pH of a 0.030 M HCl solution? 16) What is the pH of a 0.0050 M NaOH solution? 17) What is the [H+], [OH-], pH, and pOH of a 5.0 X 10-4M HNO3 solution? (Show all your work.) Some scrambled answers: 0.001, 0.23, 2.3, 9.6, 2.6, 0.125, 4.3, 12, 1.5