Energetics of Forming Ionic Bonds
advertisement

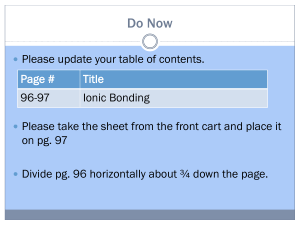
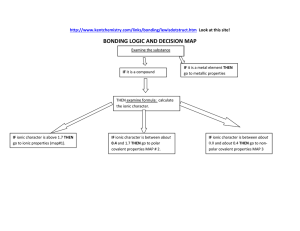
Energetics of Forming Ionic Bonds Energy Costs Mg Mg2+ + 2e O + 2e O2 H = 2200 kJ H = 900 kJ H = 3100 kJ Energy Savings Mg2+ + O2 MgO H = 3900 kJ Bottom Line Htotal = 3100 kJ – 3900 kJ = 800 kJ Ionic bonds form because the end result is a lower energy arrangement (Htotal is negative). QQ 1 2 , r where Q1 and Q2 = ion charges (this is Coulomb’s law). Note that the energy of attraction of an ionic bond Question: The ionic bond between [Mg3+][ O3] would have a larger energy of attraction due to the higher charges, but it doesn’t form. Why?