BALANCING EQUATIONS PRACTICE PROBLEMS
advertisement

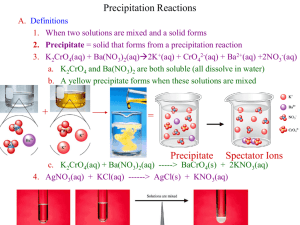
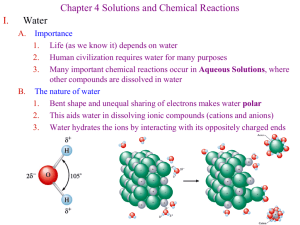
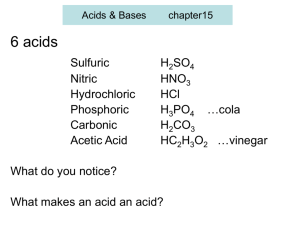
BALANCING EQUATIONS PRACTICE PROBLEMS NAME KEY Directions: Balance each of the following equations. If it is already balanced, write BALANCED after the equation. 2 Cl2 Na + NaCl SO2 CaO + N2 + 2 H2 3 2 Br2 2 K+ 2 Zn + 2 KClO3 2 5 SF4 + I 2 O5 2 CH3CHO + 2 Mn2+ + 2 KCl + 4 ZnCl + 3 H2 O2 IF5 + 2 5 SO2 HC2H3O2 OH- Mn(OH) 2 Ca(OH) 2 H2SO4 + Na2CO3 + 2 KBr O2 2 HCl 2 Balanced NH3 HCl 2 CaSO3 CaSO4 + 2 NaCl + 2 H2 O CO2 + H2 O Complete the following reactions (include Type of reaction & state of matter for the products (remember to refer to the solubility rules)): 2 C4H10(g) + 2 HCl(aq) K2CrO4(aq) + 2 AgNO3(aq) + 2 H2(g) + 13 O2(g) H2(g) 8 CO2(g) + + Cl2(g) Ba(NO3)2(aq) Cu2+(s) O2(g) 2 10 H2O(l) Combustion Decomposition BaCrO4(s) + Cu(NO3)2(aq) 2 KNO3(aq) + 2 Ag(s) Double Displacement Single Displacement H2O(l) Synthesis Give the oxidation number/ionic charge (include symbol) for the following items: S2- Sulfur Pb4+ Lead (IV) Cd2+ Cadmium Br- Bromine Rb+ Rubidium N3- Nitrogen SO42- Sulfate PO33- Phosphite NO3- Nitrate CO32- Carbonate C2H3O2- Acetate OH- Hydroxide BALANCING EQUATIONS PRACTICE PROBLEMS Write the compound formula for the following: Barium chloride BaCl2 Ammonium carbonate (NH4)2CO3 Sodium nitrate NaNO3 Tin (IV) oxide SnO2 Barium oxide BaO Write the chemical name for the following compound formulas: Fe(OH)3 Iron (III) hydroxide K3Fe(CN)6 Potassium ferricyanide Mg3(PO4)2 Magnesium phosphate NaCl Sodium Chloride KI Potassium iodide Al(IO3)3 Aluminum iodate Cu2SO4 Copper (I) sulfate For the following indicate if the reaction WILL or WILL NOT occur (refer to the activity series): Ag(s) + CuSO4(aq) No Reaction (Will Not) Mg(s) + HCl(aq) Reaction (Will) Al(s) + Pb(NO3)2(aq) Reaction (Will) Lead + Aluminum nitrate No Reaction (Will Not) Tin + Iron (III) nitrate No Reaction (Will Not) What do the following items tell us and where are they located? Coefficient: Located before the symbol, tells number of substances Subscript: located at bottom right of symbol, tells number of atoms Superscript: located at upper right of symbol, tells ionic charge/oxidation number