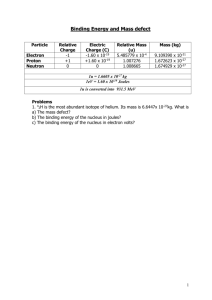
The Rutherford Experiment The Rutherford experiment
advertisement

The Rutherford Experiment The Rutherford experiment (performed by Geiger and Marsden) is famous for the unexpected observation of alpha particles bouncing off a thin sheet of gold foil. Existing models of the atom suggested it to be a uniform positive “blob” with electrons evenly distributed (known as Thomson’s Plum Pudding Model). Due to the high energy of the alpha particle it should have punched straight through. Although, the majority of the alpha particles did pass through, occasionally an alpha particle scattered backwards. Rutherford’s description was, “…it was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you.” Rutherford’s bold conjecture to explain this observation was that the atom was not a uniform blob but had a central positively charged nucleus with orbiting electrons. Furthermore based on conservation of energy calculations, Ruther was able to estimate the maximum possible size of the nucleus and based on the known estimate for the size of an atom he was able to conclude the atom was mostly empty space. Determining the maximum radius of a gold nucleus!!!! Rutherford was able to determine the maximum radius of a gold nucleus through conservation of energy. a. If the gold nucleus can be considered a sphere with a charge of +79e, determine the electric potential at a distance “r” from the nucleus. b. If the alpha particle has a charge of 2e, determine the electrical potential energy of the alpha particle at this distance “r.” c. If the alpha particle starts with a kinetic energy of 8 MeV and no potential energy determine how close in meters it can get to the gold nucleus? In other words, determine “r” for when all of the kinetic energy of the alpha particle turns to electrical potential energy. d. If the radius of the gold atom was estimated to be on the order of 10-10 m, what percent of the gold atom’s volume does the nucleus occupy and what percent is empty space (based on your calculation of the maximum possible radius)? e. If you were to make a scale model of the atom in which the nucleus at a diameter of 1 cm, what would be the diameter of your atom in meters?