Periodic Trends: Graphing Atomic Radii
advertisement

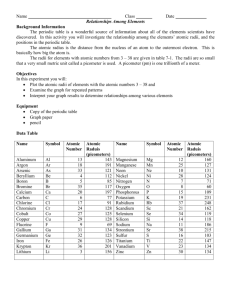
Periodic Trends: Graphing Atomic Radii The periodic table is a wonderful source of information about all the elements scientists have discovered. In this activity, you will investigate the relationship among elements’ atomic numbers, radii, and positions in the periodic table. The radii for elements with atomic numbers 3-38 are given in table 1. The radii are so small that a very small metric unit called a picometer is used. A picometer (pm) is one trillionth of a meter (1 x 10-12 m). In this activity, you will graph the atomic radii of the elements with atomic numbers 3-38 and examine the graph for repeating patterns. Table 1: Atomic Radii Name and symbol Atomic number Aluminum Al 13 Argon Ar 18 Arsenic As 33 Beryllium Be 4 Boron B 5 Bromine Br 35 Calcium Ca 20 Carbon C 6 Chlorine Cl 17 Chromium Cr 24 Cobalt Co 27 Copper Cu 29 Fluorine F 9 Gallium Ga 31 Germanium Ge 32 Iron Fe 26 Krypton Kr 36 Lithium Li 3 Atomic radius (picometers) 143 191 121 112 85 117 197 77 91 128 125 128 69 134 123 126 201 156 Name and symbol Magnesium Manganese Neon Nickel Nitrogen Oxygen Phosphorus Potassium Rubidium Scandium Selenium Silicon Sodium Strontium Sulfur Titanium Vanadium Zinc Mg Mn Ne Ni N O P K Rb Sc Se Si Na Sr S Ti V Zn Atomic number 12 25 10 28 7 8 15 19 37 21 34 14 11 38 16 22 23 30 Atomic radius (picometer) 160 127 131 124 71 60 109 231 248 162 119 118 186 215 103 147 134 134 Procedure 1. On the graph paper, label the horizontal axis with the numbers 0-38 to represent the atomic numbers of the elements you will be plotting. 2. Label the vertical axis by ten with numbers 0- 280. These numbers represent the atomic radii. 3. Plot the atomic radius for each of the elements with atomic numbers 3-38. Label each point with the corresponding element symbol. When you have finished plotting points, connect the plotted data to create a line graph. Source: Glencoe/McGraw-Hill pp. 21-22 Periodic Trends: Graphing Atomic Radii Source: Glencoe/McGraw-Hill pp. 21-22 Questions and Conclusions: 1. Look at the shape of your graph. What patterns do you observe? _________________ ____________________________________________________________________ ____________________________________________________________________ 2. What family is represented by the high peaks on your graph? ____________________ 3. What family is represented by the low peaks on your graph? _____________________ 4. What family is represented by the smaller peaks just before the high peaks in your graph?_______________________________________________________________ 5. What do you notice about the radii of the elements at the high peaks as you move from left to right on your graph? Look at your periodic table and find the element that represents each high peak. Where does each high peak begin in the periodic table? ____________________________________________________________________ ____________________________________________________________________ 6. What happens to the radii of the elements between two highest peaks? What does each of these groups of elements represent? ____________________________________________________________________ ____________________________________________________________________ ____________________________________________________________________ 7. How can graphs such as the one you made help to predict the properties of elements that have not been discovered yet? _____________________________________________ ____________________________________________________________________ ____________________________________________________________________ ____________________________________________________________________ ____________________________________________________________________ 8. How do the radii of metals in each period compare with the radii of nonmetals in that period? ____________________________________________________________________ ____________________________________________________________________ Source: Glencoe/McGraw-Hill pp. 21-22