From www.bloodjournal.org by guest on March 6, 2016. For personal use only.
Review in translational hematology
New agents that stimulate erythropoiesis
H. Franklin Bunn1
1Hematology
Division, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA
Recombinant human erythropoietin
(rhEpo) has proven to be remarkably safe
and effective for treatment of anemias,
primarily those secondary to renal disease and malignancy. Despite the worldwide use of rhEpo, concerns about its
cost, the need for frequent parenteral
administration, and the development of
anti-Epo antibodies have prompted development of improved agents to stimulate
erythropoiesis. Three strategies appear
to be particularly promising. The half-life
of Epo in the circulation can be prolonged
by the addition of N-linked carbohydrate
groups, by formation of adducts with
polyethylene glycol, and by preparation
of Epo multimers. Second, mimetic peptides can effectively trigger signal transduction at the Epo receptor, thereby
boosting red-cell production. Finally, the
hypoxia inducible transcription factor
(HIF) can be pharmacologically induced
by oral agents, resulting in enhanced
expression not only of endogenous Epo
but also of other genes important in the
regulation of erythropoiesis. (Blood. 2007;
109:868-873)
© 2007 by The American Society of Hematology
Introduction
Recombinant human erythropoietin (rhEpo) is arguably the most
successful therapeutic agent spawned thus far by the advent of
molecular genetic technology. Since the initial reports 20 years ago
demonstrating cure of the anemia of chronic renal failure,1,2 well
over a million patients with uremia have been effectively treated,
with remarkably few adverse side effects or complications. Amgen,
the world’s largest biotechnology company, has sold about $22
billion worth of its rhEpo product Epogen and, more recently, about
$7 billion worth of darbepoetin alfa (Aranesp), a derivative of
rhEpo with a longer half-life (described under “Modifications of
full-length rhEpo that prolong plasma survival”). The use of rhEpo
has further expanded following its approval by the Food and Drug
Administration for the treatment of anemias associated with cancer
and AIDS. In addition, rhEpo has been used effectively in the
treatment of anemia in myelodysplasia and the anemia of prematurity as well in surgical patients, in both the preoperative and
postoperative periods.
Despite the enormous success of rhEpo therapy, there is concern
among patients and their physicians that it is costly (often
⬎ $10 000/year). The emergence on the market of a less expensive
generic rhEpo has been delayed by extended and sequential
patents. In addition, many patients object to parenteral administration as often as 3 times a week. Finally, considerable anxiety was
engendered from a spate of reports, from 1998 to 2004, of the
development of severe pure red-cell aplasia in European patients
treated with rhEpo.3,4 However, this complication is now encountered only rarely and was probably caused by defective formulation
rather than inherent antigenicity of rhEpo.
Because of these concerns, there is considerable impetus in the
pharmaceutical industry to develop less costly agents that match
rhEpo in safety and efficacy but are more easily administered, and
nonantigenic. This article provides information on new erythropoietic-stimulating agents that aim to meet one or more of these
criteria. In order to understand the rationale underlying the design
of these agents, it is worthwhile to first briefly review the
Submitted August 14, 2006; accepted August 25, 2006. Prepublished online
as Blood First Edition Paper, October 10, 2006; DOI 10.1182/blood-2006-
868
interaction of Epo with its receptor (EpoR) as well as the regulation
of the Epo gene.
Epo–EpoR interaction
Epo is a member of an extensive cytokine family that includes
growth hormone, prolactin, interleukins 2 through 7, granulocyte
colony-stimulating factor (G-CSF), granulocyte macrophage (GM)–
CSF, M-CSF, and others. Although there is very weak sequence
homology among these proteins, they have similar numbers of
exons and have either been demonstrated or are predicted to fold
into a compact globular structure consisting of 4 amphipathic
␣-helical bundles (see review by Kaushansky and Karplus5).
Extensive site-directed mutagenesis studies indicate that Epo has 2
domains, primarily located on Helix C and Helix D, that are
necessary for binding to the Epo receptor (EpoR).6-10 Subsequent
x-ray diffraction analysis of the complex between Epo and the
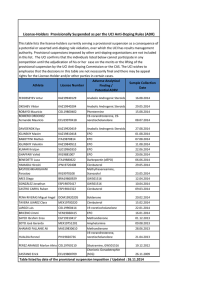
extracellular ligand-binding portion of EpoR (Figure 1A) is
consistent with these mutagenesis studies but provides much more
detailed information on the binding sites.11 The presence of 2
functionally important domains supports the notion that a single
Epo molecule brings 2 EpoR molecules together, thereby initiating
a signal transduction cascade. However, x-ray structural analysis of
the unliganded extracellular EpoR surprisingly revealed that it too
is dimeric but has a very different conformation.12 As shown in
Figure 1B, in the absence of Epo the arms of the dimeric
extracellular EpoR that attach to the transmembrane domains are
splayed apart by a distance of 73 Å. This distance prevents JAK2,
which binds to the cytosolic domain of each EpoR polypeptide,
from phosphorylating its dimeric partner, and thus the signal
transduction cascade is not initiated. Upon binding of Epo, the
conformation change in the extracellular domain of dimeric EpoR
shortens the transmembrane distance to 39 Å, allowing autophosphorylation and activation of JAK2, thereby triggering the signal
transduction cascade necessary for Epo’s biologic activity.13
08-019083.
© 2007 by The American Society of Hematology
BLOOD, 1 FEBRUARY 2007 䡠 VOLUME 109, NUMBER 3
From www.bloodjournal.org by guest on March 6, 2016. For personal use only.
BLOOD, 1 FEBRUARY 2007 䡠 VOLUME 109, NUMBER 3
ERYTHROPOIESIS-STIMULATING AGENTS
869
Figure 1. Binding of Epo to its homodimeric receptor and initiation of signal transduction. (A) Crystal structure of the complex of Epo with 2 extracellular domains of
EpoR (from Syed et al).11 Red cylinders denote ␣-helices and green ribbons denote -sheets. For detailed information on interacting sites in the complex see Syed et al.11 (B)
Schematic representation of the conformational change imposed on the dimeric Epo receptor (EpoR) upon binding to Epo. The close proximity off the cytosolic domains of the
dimeric EpoR enables autophosphorylation of JAK2 and the initiation of signal transduction. Modified from Remy et al.13
Regulation of the Epo gene
Plasma Epo is produced primarily in the kidney, and to a lesser
extent in the liver. The expression of Epo mRNA and protein is
regulated primarily at the transcriptional level. The most physiologically important and best understood stimulus for Epo production is
hypoxia. Clinicians are familiar with the progessive logarithmic
rise in plasma Epo levels in patients with anemias of increasing
severity. The marked enhancement of EPO transcription at low
oxygen tension is mediated through the hypoxia-inducible transcription factor HIF.14-16 As shown in Figure 2A, this ␣ heterodimer
binds to a cognate hypoxia response element in a crucial enhancer
located just 3⬘ to EPO’s polyadenylation site (3⬘ Enh). HIF is
expressed in nearly all cells and organs and serves as the master
orchestrator of oxygen-dependent expression of a number of
physiologically important genes. There are 3 highly homologous
Figure 2. Hypoxic induction of the Epo gene. (A) The Epo gene has 5
exons, represented by rectangles. The coding regions are shown in black.
The 3⬘ enhancer binds to HNF-4 (hepatic nuclear factor 4) and, in hypoxic
cells, to HIF (hypoxia inducible factor). These 2 transcription factors bind
cooperatively with the adaptor protein p300. This complex interacts with
the Epo promoter, thereby enabling initiation of transcription. Upstream
kidney inducible elements (KIEs) are required for enhanced renal expression of Epo. (B) The oxygen-sensing mechanism responsible for the
oxygen-dependent degradation of HIF-␣. In well-oxygenated cells, HIF-1␣
protein undergoes oxygen- and iron-dependent hydroxylation at proline
564 and proline 402 (not shown). These posttranslational modifications
enable the von Hipple Lindau protein (pVHL) to bind specifically to HIF-␣,
thereby providing a docking site for the E3 ubiquitin ligase (UL). UL is
required for ubiquitylation of HIF-␣, resulting in uptake by the proteasome
and proteolytic degradation. At low oxygen tension, HIF-␣ is not hydroxylated and therefore escapes degradation. HIF-␣ can be activated in
oxygenated cells by inhibitors of prolyl hydroxylase (PH) or by iron
chelation.
HIF␣ subunits with differential tissue specificity and affinity for
cognate hypoxia response elements. It is not clear whether the EPO
gene is regulated primarily by HIF-1␣17 or HIF-2␣.18
At normal oxygen tension the ␣-subunit of HIF is posttranslationally
modified by proline hydroxylation and ubiquitylation, causing it to be
rapidly degraded (Figure 2B). Only in hypoxic cells can HIF-␣ survive,
allowing nuclear translocation, ␣ dimer assembly, and induction (or
repression) of gene expression. The robust hypoxic induction of Epo in
kidney and liver is due in part to cooperation in the 3⬘ enhancer between
HIF and HNF-4, a nuclear receptor that is preferentially expressed in
these organs (Figure 2A). These 2 transcription factors interact cooperatively with the transcriptional adaptor p300. Moreover, as shown in
Figure 2A, the high renal level of Epo expression also depends on an
as-yet-uncharacterized cis element in the Epo gene, 9 kb to 14 kb
upstream of the promoter.
From www.bloodjournal.org by guest on March 6, 2016. For personal use only.
870
BUNN
Modifications of full-length rhEpo that
prolong plasma survival
The initial rhEpo and that most commonly administered to date is
the full-length unmodified Epo polypeptide, which has an amino
acid sequence identical to that of endogenous Epo. Both contain
about 40% carbohydrate, although the composition and arrangement of the sugar moieties differ slightly. Both have similar
turnover times in the plasma with a t1/2 of about 7 to 8 hours. In
contrast, nonglycosylated Epo is very rapidly cleared from the
circulation. However, its in vitro erythroid activity and its nonerythroid biologic activity are at least as great as that of native Epo.
Realizing that asparagine(N)-linked glycosylation is an important contributor to the prolonged survival of Epo in the circulation,
scientists at Amgen reasoned that engineering additional N-linked
sites into the Epo polypeptide might result in a product with an
even longer half-life. The product with the most efficacy, darbepoetin alfa (Aranesp), has multiple mutations that create 2 N-linked
glycosylation sites, above and beyond the 3 normally present in
endogenous Epo. These modifications result in a 3-fold increase in
plasma half-life,19 enabling less frequent administration. As mentioned in “Introduction,” this drug is currently in widespread use. In
patients with chronic kidney disease, darbepoetin alfa is generally
given once every 2 weeks, but even monthly administration is
effective.20 In hemodialysis patients the drug is usually given
intravenously but in predialysis patients subcutaneous administration, at equivalent dosing, is more convenient and equally effective.21 Darbepoetin alfa is also widely used in cancer patients
undergoing chemotherapy, and is comparable to rhEpo in significantly reducing transfusion requirements.22,23 A 3-week dosing
interval allows synchronization with most chemotherapy regimens.24 Although there is much less overall experience and
reported information in patients with myelodysplasia, darbepoetin
alfa is at least as effective as rhEpo in improving anemia and
lowering transfusion requirements in a substantial fraction of
patients, particularly those with relatively low endogenous plasma
Epo levels.25,26 A single dose of darbepoetin alfa has also shown to
be effective in the treatment of anemic preterm neonates.27
Three other strategies have been used to prolong the half-life of
Epo in the circulation. Recombinant dimeric Epo linked via a
flexible peptide bridge28 and rhEpo chemically crosslinked via free
sulfhydryl groups to form dimers and trimers29 were both found
to have enhanced specific bioactivity and markedly prolonged
half-life. Clinical trials have not yet been carried out on these
dimeric Epo’s.
Scientists at Gryphon Therapeutics have developed synthetic
erythropoiesis protein (SEP), which is a fully synthetic 50.8-kDa
macromolecule of uniform structure composed of 166-amino-acid
polypeptide with a sequence similar to but not identical to that of
native Epo. Negatively charged noncarbohydrate precision-length
branched polymers have been attached to this protein at 2 sites by
chemical ligation.30 SEP has in vitro erythropoietic activity that is
considerably greater than that of rhEpo, probably owing to its
having an approximately 2.5 prolongation of plasma half-life.
The conjugation of rhEpo to polyethylene glycol results in a
product that also has prolonged survival in the circulation.31 Two
companies, Prolong Pharmaceuticals and Roche, have developed
structurally distinct PEGylated Epo products for therapeutic use.
Information is available only on the Roche product.32 The Roche
product CERA (Continuous Erythropoietin Receptor Activator) is a
60-kDa molecule, twice the mass of Epo. A methoxy-polyethylene
BLOOD, 1 FEBRUARY 2007 䡠 VOLUME 109, NUMBER 3
glycol polymer is incorporated into Epo at the N-terminal amino as
well as the ⑀-amino of lysine 52 or lysine 45. This modification
markedly prolongs the product’s half-life in the circulation to about
135 hours in humans after either intravenous or subcutaneous
administration. Surface plasmon resonance (Biacore) studies indicate that CERA binds to EpoR more slowly than Epo and that its
dissociation (“off”) rate is faster. Thus CERA can trigger the Epo
signal transduction cascade without being internalized and has
more sustained biologic activity. A single administration of CERA
to healthy human volunteers results in a dose-dependent rise in
reticulocytes that peaks at about day 7. The agent has few if any
significant adverse effects in healthy individuals or in over a
thousand patients. No antierythropoietin antibodies have developed
to date following CERA treatment. Phase 2 studies have shown
impressive erythropoietic responses in patients with chronic renal
failure as well as in those with multiple myeloma and non-Hodgkin
lymphoma. A phase 3 study is currently under way in patients with
non-Hodgkin lymphoma.
In principle, Epo’s that have been engineered to have a long
half-life should require administration of less protein product and
therefore the cost for maintenance therapy should be less. Unfortunately, this is not the case for predialysis patients treated with
darbepoetin alfa.33
Small molecule Epo mimetics
In 1996 scientists from Scripps, Affimax, and Johnson Pharmaceutical Research Institute screened a peptide phage library to search
for novel sequences that bound to EpoR and possessed biologic
activity. Their strategy of using a Cys-X8-Cys insert that could be
released by thrombin cleavage resulted in generation of decapeptides capable of dimerizing by means of disulphide bonds. Because
of symmetry considerations, the dimeric peptide afforded a greater
chance of binding to the dimeric EpoR, thereby enabling triggering
of the signal transduction cascade. They found a sequence,
CRIGPITWVC, that bound weakly to EpoR (Kd ⬃10 mM) and was
used as a template for adding random flanking residues as well as
internal mutagenesis. After multiple rounds of further screening,
they isolated and identified a 20-amino-acid peptide (GGTYSCHFGPLTWVCKPQGG) that bound to EpoR with a Kd of 200 nM and
had biologic activity both in vitro and in vivo.34 X-ray analysis of
the peptide bound to the extracellular domain of EpoR revealed, for
the first time, the dimeric structure of EpoR and demonstrated the
docking sites of the dimeric peptide.35 The sequence of this peptide
bears no resemblance whatever to that of Epo. However, this
strategy offered the opportunity for further structural modifications
that might raise its affinity to EpoR approximately 1000-fold,
comparable to that of native or rhEpo.
Even if an Epo mimetic peptide had affinity for EpoR close to
that of native Epo, its low molecular weight would probably result
in rapid urinary excretion, and therefore it would be unlikely to
serve as practical or useful therapy. This concern has been
addressed by scientists at Affymax, who have developed another
peptide with no sequence homology to Epo but with EpoR
specificity. This peptide has been PEGylated in order to enhance its
stability and prolong its half-life in the circulation. The product,
Hematide, has biologic activity both in vitro and in vivo.36 This
agent has been shown to be safe in a cohort of 28 healthy human
volunteers. One time intravenous administration stimulated erythropoiesis in a dose-dependent manner, with a boost in hemoglobin
levels that lasted for at least one month.37 Preliminary results
From www.bloodjournal.org by guest on March 6, 2016. For personal use only.
BLOOD, 1 FEBRUARY 2007 䡠 VOLUME 109, NUMBER 3
indicate that Hematide is also safe and effective in the treatment of
patients with chronic kidney disease.38 Single doses resulted in
sustained increments of hemoglobin levels with a return to baseline
at approximately 43 days. Interestingly, in comparison to healthy
volunteers, a given increment in hemoglobin was achieved in
patients with renal failure at half the dose. Hematide is now being
evaluated in phase 2 studies both in renal patients and in patients
with cancer.
Activation of HIF
As explained in the “Introduction,” in the absence of anemia or
other causes of hypoxia, Epo expression in the kidney and liver is
suppressed by the oxygen-dependent degradation of the ␣-subunit
of HIF, mediated by prolyl hydroxylation (Figure 2B). Three HIF␣
prolyl hydroxylases (PHDs) have been cloned and characterized.
PHD2 appears to play the dominant biologic role. Inhibition of
HIF␣ prolyl hydroxylation results in increased levels of HIF even
in oxygenated cells, and therefore should enhance expression of
Epo as well as other HIF responsive genes. Prolyl hydroxylation
depends on the presence of oxygen, iron and the cofactor 2-oxoglutarate (Figure 2B). N-oxalylglycine, an analog of 2-oxoglutarate, is
a potent inhibitor of prolyl hydroxylation and therefore induces
HIF activation.39 During the past decade, scientists at Fibrogen
have developed a large series of other inhibitors of prolyl hydroxylases with the aim of preventing formation of hydroxyproline that is
essential for stable triple helical collagen. This group of compounds was screened for the ability to up-regulate Epo expression
in the Hep3B hepatoma cell line. Several lead compounds markedly induced Epo without any effect on vascular endothelial growth
factor, another gene that is normally HIF responsive. One compound (FG-4487) strongly induced the accumulation of HIF-1␣
and HIF-2␣ in renal tubular and peritubular cells along with HIF
target gene expression in a rat model of acute ischemic renal
failure.40 Of considerable interest is the finding that 2 of their lead
compounds, FG-2216 and FG-4592, up-regulate other genes besides Epo that are important in erythropoiesis including EpoR,
transferrin, transferrin receptor, ferroportin, and the divalent metal
transporter 1.41
Oral administration of either FG-2216 or FG-4592 was more
effective than parenteral darbepoetin in correcting anemia and
reducing hepcidin expression in a rat model of inflammation.42 In
healthy human volunteers, dose escalation of another lead compound, FG-2216, resulted in a graded increase in hemoglobin
levels with only a modest increase in plasma Epo. A phase 2
dose-escalation study of FG-2216 in patients with chronic kidney
disease revealed stimulation of erythropoiesis comparable to
standard rhEpo/darbepoetin therapy but, importantly, with plasma
Epo levels an order of magnitude lower.43 Thus, it is likely that the
coordinated up-regulation of the above-mentioned genes involved
in iron mobilization contributes in a major way to the enhanced
erythropoiesis. This phenomenon is akin to Chuvash polycythemia
in which an Arg200Trp mutation in the VHL protein (Figure 2B)
results in constitutive HIF activation and in a marked increase in
red-cell mass but often only modestly increased plasma
erythropoietin.44
A major concern with the use of this type of drug is whether
adverse effects might arise owing to activation or suppression of
some of the many other genes known to be regulated by HIF. For
example, might the drug trigger unwanted vascular neogenesis?
Even more concerning is the possibility that the drug may induce or
ERYTHROPOIESIS-STIMULATING AGENTS
871
aggravate neoplasia. The fact that HIF is constitutively expressed
in many cancers, particularly metastatic ones,45 raises the specter of
enhanced tumor growth in the presence of a drug that activates HIF.
These concerns seem to be at least partially addressed by the
finding that FG-4592 and FG-2216 are remarkably specific for
up-regulation of Epo and a family of other genes that regulate
erythropoiesis. Indeed, in 5 different mouse models of lung or
colon cancer, FG-2216 corrected the anemia but tumor growth was
not enhanced and may have been retarded.46 The specificity of
these agents may be based on differential inhibition of the 3 PHDs
and/or differential activation of the 3 HIF␣ subunits. Clinical trials
are under way to assess the safety and efficacy of these agents in
different types of anemias. In addition to inhibitors of prolyl
hydroxylase, HIF may also be activated by iron chelation, both in
vitro and in vivo.47 It is worth noting that patients with transfusional iron overload on chronic iron chelation therapy, like those
with Chuvash polycythemia, have not evinced any apparent
increase in tumor development.
The development of a potentially inexpensive small-molecule
oral agent with erythropoietic specificity offers obvious therapeutic advantages.
Concerns and caveats
During the development of rhEpo it was initially believed that the
expression of EpoR was limited to erythroid progenitors and
therefore Epo therapy would have a high degree of specificity with
few if any nonerythropoietic effects. However, it has become
increasingly apparent that EpoR is present on a wide range of cells
and that high (pharmacologic) doses may result in diverse sequelae
at sites outside of the erythron.48 Possible beneficial effects include
protection of the central and peripheral nervous system, myocardium, and other tissues against injury. Modification of Epo by
removal of sialic acid, which greatly shortens its half-life in the
circulation, has been shown to be neuroprotective in models of
cerebral ischemia, spinal cord compression, and sciatic nerve
injury with no significant stimulation of erythropoiesis.49 In like
manner, Epo modified by carbamylation lacks erythropoietic
activity but prevents cardiomyocyte loss in a model of myocardial
infarction.50 In nonerythroid cells, EpoR is present in much lower
abundance. It is likely that in these cells Epo and the modified
Epo’s mentioned above bind to a heterodimer consisting of EpoR
and the common cytokine  receptor.51
The potential and proven beneficial nonerythroid effects of Epo
notwithstanding, there is concern that chronic administration of
pharmacologic doses of rhEpo could have several types of deleterious consequences including neovascularization, thrombosis, and
enhancement of cancer growth.
Among its many nonerythropoietic effects, Epo stimulates
proliferation and migration of vascular endothelial cells in vitro
and promotes angiogenesis.52,53 Epo may contribute to the pathogenesis of proliferative diabetic retinopathy. Levels of both Epo and
vascular endothelial growth factor (VEGF) were markedly higher
in the vitreous fluid of these patients than in those with nondiabetic
ocular disease.54 Epo levels were more strongly associated with
proliferative vessel disease compared with VEGF. Inhibition of
both Epo and VEGF greatly retarded the growth of bovine retinal
microvascular cells.
During the early days of treating hemodialysis patients with
rhEpo, one of the uncommon complications was thrombosis,
particularly in arteriovenous shunts or fistulas. This problem was
From www.bloodjournal.org by guest on March 6, 2016. For personal use only.
872
BLOOD, 1 FEBRUARY 2007 䡠 VOLUME 109, NUMBER 3
BUNN
thought to be due to excessive dosing, with hematocrit levels rising
to the mid- or high 40s. However, rhEpo may pose a thrombotic
risk independent of its effect on the red-cell mass. Administration
of rhEpo to dogs resulted in a decline in platelet count but enhanced
platelet reactivity,55 and promoted development of thrombus in
those with an arteriovenous shunt.56 In healthy human volunteers,
intravenous administration of rhEpo (100 U/kg or 500 U/kg)
resulted in a 10% to 20% increase in platelet count as well as
activation of both platelets and the endothelium.56 However, the
rise in platelets may be due in part to induction of iron deficiency
owing to the increase in red-cell mass.58 Cancer patients are
inherently predisposed to thrombosis. This complication may be
enhanced in those treated with rhEpo. In a retrospective review of
9353 cancer patients who participated in 57 trials, the administration of rhEpo or darbepoetin alfa significantly reduced the need for
red-cell transfusions but increased thrombo-embolic events with a
relative risk ratio of 1.67.23
It is not surprising that the many organs and tissues that express
EpoR include a wide variety of human malignancies, both solid
tumors and leukemias.48 In some animal tumor models, inhibition
of Epo binding to EpoR has resulted in tumor regression.59,60 A
multicenter trial of patients with breast cancer treated with
chemotherapy was terminated early because of increased mortality
in the first 4 months of those also treated with rhEpo.61 In a
double-blind, placebo-controlled study of 351 patients with head
and neck cancer, those receiving rhEpo had higher hemoglobin
levels than those given placebo, but had significantly greater cancer
progression and lower survival.62 In contrast to these studies,
Hedenus et al63 found that darbepoetin alfa therapy had no effect on
tumor progression (overall survival or disease-free survival) in 314
patients with lung cancer and 344 patients with lymphoma.
Fear of adverse effects from high doses of rhEpo is somewhat
allayed by the fact that patients with severe chronic anemias do not
appear to have complications that can plausibly be attributed to
levels of circulating Epo that are often 100 times normal. Concern
about nonerythroid effects of Epo therapy would be minimized by
the agents described above that activate HIF and achieve sustained
and efficient enhancement of erythropoiesis despite only a modest
increase in plasma Epo.
Conclusions
A reasonably full understanding of the regulation of the Epo gene
as well as the interaction of Epo with EpoR has permitted fruitful
exploration of rational strategies for the development of new
erythropoietic agents that match rhEpo in safety and efficacy but
are easier to administer, better tolerated, and, hopefully, much less
costly. Within the next few years one or more of these agents are
likely to be in widespread use around the world.
Authorship
Conflict-of-interest disclosure: The author declares no competing
financial interests.
Correspondence: H. Franklin Bunn, Hematology Division,
Brigham and Women’s Hospital, Harvard Medical School, Boston,
MA 02115; e-mail: hfbunn@rics.bwh.harvard.edu.
References
1. Winearls CG, Oliver DO, Pippard MJ, Reid C,
Downing MR, Cotes PM. Effect of human erythropoietin derived from recombinant DNA on the
anaemia of patients maintained by chronic haemodialysis. Lancet. 1986;2:1175-1178.
2. Eschbach JW, Ergie JC, Downing MR, Browne
JK, Adamson JW. Correction of the anemia of
endstage renal disease with recombinant human
erythropoietin. N Engl J Med. 1987;316:73-78.
3. Casadevall N, Nataf J, Viron B, et al. Pure redcell aplasia and antierythropoietin antibodies in
patients treated with recombinant erythropoietin.
N Engl J Med. 2002;346:469-475.
4. Bennett CL, Luminari S, Nissenson AR, et al.
Pure red-cell aplasia and epoetin therapy. N Engl
J Med. 2004;351:1403-1408.
5. Kaushansky K, Karplus PA. Hematopoietic
growth factors: understanding functional diversity
in structural terms. Blood. 1993;82:3229-3240.
6. Grodberg J, Davis KL, Sytkowski AJ. Alanine
scanning mutagenesis of human erythropoietin
identifies four amino acids which are critical for
biological activity. Euro J Biochem. 1993;218:
597-601.
7. Wen D, Boissel J-P, Showers M, Ruch BC, Bunn
HF. Erythropoietin structure-function relationships: identification of functionally important domains. J Biol Chem. 1994;269:22839-22846.
8. Elliott S, Lorenzini T, Chang D, et al. Fine-structure epitope mapping of anti-erythropoietin monoclonal antibodies reveals a model of recombinant
human erythropoietin protein structure. Blood.
1996;87:2702-2713.
9. Elliott S, Lorenzini T, Chang D, Barzilay J, Delorme E. Mapping of the active site of recombinant human erythropoietin. Blood. 1997;89:493502.
10. Qiu H, Belanger A, Yoon HW, Bunn HF. Homodimerization restores biological activity to an
inactive erythropoietin mutant. J Biol Chem.
1998;273:11173-11176.
11. Syed RS, Reid SW, Li C, et al. Efficiency of signalling through cytokine receptors depends critically on receptor orientation. Nature. 1998;395:
511-516.
12. Livnah O, Stura EA, Middleton SA, Johnson DL,
Jolliffe LK, Wilson IA. Crystallographic evidence
for preformed dimers of erythropoietin receptor
before ligand activation. Science. 1999;283:987990.
13. Remy I, Wilson IA, Michnick SW. Erythropoietin
receptor activation by a ligand-induced conformation change. Science. 1999;283:990-993.
14. Huang LE, Bunn HF. Hypoxia-inducible factor and
its biomedical relevance. J Biol Chem. 2003;278:
19575-19578.
15. Schofield CJ, Ratcliffe PJ. Oxygen sensing by
HIF hydroxylases. Nat Rev Mol Cell Biol. 2004;5:
343-354.
16. Fandrey J. Oxygen-dependent and tissue-specific regulation of erythropoietin gene expression.
Am J Physiol Regul Integr Comp Physiol. 2004;
286:R977–R988.
17. Yu AY, Shimoda LA, Iyer NV, et al. Impaired physiological responses to chronic hypoxia in mice
partially deficient for hypoxia-inducible factor 1alpha. J Clin Invest. 1999;103:691-696.
18. Rosenberger C, Mandriota S, Jurgensen JS, et
al. Expression of hypoxia-inducible factor-1alpha
and -2alpha in hypoxic and ischemic rat kidneys.
J Am Soc Nephrol. 2002;13:1721-1732.
19. Macdougall IC, Gray SJ, Elston O, et al. Pharmacokinetics of novel erythropoiesis stimulating protein compared with epoetin alfa in dialysis patients. J Am Soc Nephrol. 1999;10:2392-2395.
20. Ling B, Walczyk M, Agarwal A, Carroll W, Liu W,
Brenner R. Darbepoetin alfa administered once
monthly maintains hemoglobin concentrations in
patients with chronic kidney disease. Clin Nephrol. 2005;63:327-334.
21. Cervelli MJ, Gray N, McDonald S, Gentgall MG,
Disney AP. Randomized cross-over comparison
of intravenous and subcutaneous darbepoetin
dosing efficiency in haemodialysis patients. Nephrology (Carlton). 2005;10:129-135.
22. Glaspy J, Vadhan-Raj S, Patel R, et al. Randomized comparison of every-2-week darbepoetin
alfa and weekly epoetin alfa for the treatment of
chemotherapy-induced anemia: the 20030125
Study Group Trial. J Clin Oncol. 2006;24:22902297.
23. Bohlius J, Wilson J, Seidenfeld J, et al. Recombinant human erythropoietins and cancer patients:
updated meta-analysis of 57 studies including
9353 patients. J Natl Cancer Inst. 2006;98:708714.
24. Siddiqui MA, Keating GM. Darbepoetin alfa: a
review of its use in the treatment of anaemia in
patients with cancer receiving chemotherapy.
Drugs. 2006;66:997-1012.
25. Stasi R, Abruzzese E, Lanzetta G, Terzoli E,
Amadori S. Darbepoetin alfa for the treatment of
anemic patients with low- and intermediate-1-risk
myelodysplastic syndromes. Ann Oncol. 2005;16:
1921-1927.
26. Mannone L, Gardin C, Quarre MC, et al. Highdose darbepoetin alpha in the treatment of anaemia of lower risk myelodysplastic syndrome results of a phase II study. Br J Haematol. 2006;
133:513-519.
27. Warwood TL, Ohls RK, Wiedmeier SE, et al.
Single-dose darbepoetin administration to anemic
preterm neonates. J Perinatol. 2005;25:725-730.
28. Sytkowski AJ, Lunn ED, Risinger MA, Davis KL.
An erythropoietin fusion protein comprised of
identical repeating domains exhibits enhanced
From www.bloodjournal.org by guest on March 6, 2016. For personal use only.
BLOOD, 1 FEBRUARY 2007 䡠 VOLUME 109, NUMBER 3
biological properties. J Biol Chem. 1999;274:
24773-24778.
29. Sytkowski AJ, Lunn ED, Davis KL, Feldman L,
Siekman S. Human erythropoietin dimers with
markedly enhanced in vivo activity. Proc Natl
Acad Sci U S A. 1998;95:1184-1188.
30. Kochendoerfer GG, Chen SY, Mao F, et al. Design and chemical synthesis of a homogeneous
polymer-modified erythropoiesis protein. Science.
2003;299:884-887.
31. Jolling K, Ruixo JJ, Hemeryck A, Piotrovskij V,
Greway T. Population pharmacokinetic analysis
of pegylated human erythropoietin in rats.
J Pharm Sci. 2004;93:3027-3038.
32. Macdougall IC. CERA (continuous erythropoietin
receptor activator): a new erythropoiesis-stimulating agent for the treatment of anemia. Curr Hematol Rep. 2005;4:436-440.
33. Papatheofanis FJ, McKenzie RS, Mody SH, Suruki RY, Piech CT. Dosing patterns, hematologic
outcomes, and costs of erythropoietic agents in
predialysis chronic kidney disease patients with
anemia. Curr Med Res Opin. 2006;22:837-842.
34. Wrighton NC, Farrell FX, Chang R, et al. Small
peptides as potent mimetics of the protein hormone erythropoietin. Science. 1996;273:458-463.
35. Livnah O, Stura EA, Johnson DL, et al. Functional
mimicry of a protein hormone by a peptide agonist: the EPO receptor complex at 2.8 A. Science.
1996;273:464-471.
36. Woodburn KW, Fan Q, Leuther KK, et al. Preclinical evaluation of Hematide, a novel erythropoietin
receptor agonist for the treatment of anemia
caused by kidney disease [abstract]. Blood. 2004;
104. Abstract no. 2094.
37. Stead RB, Lambert J, Wessels D, et al. Evaluation of the safety and pharmacodynamics of Hematide, a novel erythropoietic agent, in a phase
1, double-blind, placebo-controlled, dose escalation study in healthy volunteers. Blood. 2006;108:
1830-1834.
38. Duliege A-M, Macdougall I, Duncan N, et al. Hematide, a synthetic peptide-based erythropoiesis
stimulating agent (ESA) demonstrates erythropoietic activity in a phase 2 single dose escalating
study in patients with chronic kidney disease
(CKD) [abstract]. Blood. 2005;106. Abstract no.
3532.
39. Epstein AC, Gleadle JM, McNeill LA, et al. C. elegans EGL-9 and mammalian homologs define a
family of dioxygenases that regulate HIF by prolyl
hydroxylation. Cell. 2001;107:43-54.
40. Bernhardt WM, Campean V, Kany S, et al. Preconditional activation of hypoxia-inducible factors
ameliorates ischemic acute renal failure. J Am
Soc Nephrol. 2006;17:1970-1978.
41. Liu DY, Neff TB, Guenzler V, et al. Novel and beneficial pharmacodynamic properties of endogenous EPO and ‘complete erythropoiesis’ induced
by selective HIF prolyl hydroxylase inhibitors [abstract]. J Am Soc Nephrol. 2005;16:761A.
42. Langsetmo I, Nichols B, Seeley T, et al. FG-2216
corrects anemia and improves iron utilization in a
rat model of anemia of chronic disease: comparison to darbepoetin [abstract]. J Am Soc Nephrol.
2005;16:481A.
43. Günzler V, Muthukrishnan E, H.H. Neumayer
KS, et al. FG-2216 increases hemoglobin concentration in anemic patients with chronic kidney disease [abstract]. J Am Soc Nephrol.
2005;16:758A.
ERYTHROPOIESIS-STIMULATING AGENTS
873
receptor. Proc Natl Acad Sci U S A. 2004;101:
14907-14912.
52. Anagnostou A, Lee ES, Kessimian N, Levinson
R, Steiner M. Erythropoietin has a mitogenic and
positive chemotactic effect on endothelial cells.
Proc Natl Acad Sci U S A. 1990;87:5978-5982.
53. Anagnostou A, Liu Z, Steiner M, et al. Erythropoietin receptor mRNA expression in human endothelial cells. Proc Natl Acad Sci U S A. 1994;91:
3974-3978.
54. Watanabe D, Suzuma K, Matsui S, et al. Erythropoietin as a retinal angiogenic factor in proliferative diabetic retinopathy. N Engl J Med. 2005;353:
782-792.
55. Wolf RF, Peng J, Friese P, Gilmore LS, Burstein
SA, Dale GL. Erythropoietin administration increases production and reactivity of platelets in
dogs. Thromb Haemost. 1997;78:1505-1509.
44. Ang SO, Chen H, Hirota K, et al. Disruption of
oxygen homeostasis underlies congenital Chuvash polycythemia. Nat Genet. 2002;32:614-621.
56. Wolf RF, Gilmore LS, Friese P, Downs T, Burstein
SA, Dale GL. Erythropoietin potentiates thrombus
development in a canine arterio-venous shunt
model. Thromb Haemost. 1997;77:1020-1024.
45. Zhong H, De Marzo AM, Laughner E, et al. Overexpression of hypoxia-inducible factor 1alpha in
common human cancers and their metastases.
Cancer Res. 1999;59:5830-5835.
57. Stohlawetz PJ, Dzirlo L, Hergovich N, et al. Effects of erythropoietin on platelet reactivity and
thrombopoiesis in humans. Blood. 2000;95:29832989.
46. Seeley TW, Langsetmo I, Stephenson R, et al.
FG-2216: tumor progression studies and correction of anemia of chronic disease in xenograft
models [abstract]. J Am Soc Nephrol. 2005;16:
481A.
47. Wang GL, Semenza GL. Desferrioxamine induces
erythropoietin gene expression and hypoxiainducible factor 1 DNA-binding activity: implications for models of hypoxia signal transduction.
Blood. 1993;82:3610-3615.
48. Jelkmann W, Wagner K. Beneficial and ominous
aspects of the pleiotropic action of erythropoietin.
Ann Hematol. 2004;83:673-686.
49. Erbayraktar S, Grasso G, Sfacteria A, et al. Asialoerythropoietin is a nonerythropoietic cytokine
with broad neuroprotective activity in vivo. Proc
Natl Acad Sci U S A. 2003;100:6741-6746.
50. Fiordaliso F, Chimenti S, Staszewsky L, et al. A
nonerythropoietic derivative of erythropoietin protects the myocardium from ischemia-reperfusion
injury. Proc Natl Acad Sci U S A. 2005;102:20462051.
51. Brines M, Grasso G, Fiordaliso F, et al. Erythropoietin mediates tissue protection through an
erythropoietin and common beta-subunit hetero-
58. Loo M, Beguin Y. The effect of recombinant human erythropoietin on platelet counts is strongly
modulated by the adequacy of iron supply. Blood.
1999;93:3286-3293.
59. Yasuda Y, Musha T, Tanaka H, et al. Inhibition of
erythropoietin signalling destroys xenografts of
ovarian and uterine cancers in nude mice. Br J
Cancer. 2001;84:836-843.
60. Arcasoy MO, Amin K, Karayal AF, et al. Functional significance of erythropoietin receptor expression in breast cancer. Lab Invest. 2002;82:
911-918.
61. Leyland-Jones B. Breast cancer trial with erythropoietin terminated unexpectedly. Lancet Oncol.
2003;4:459-460.
62. Henke M, Laszig R, Rube C, et al. Erythropoietin
to treat head and neck cancer patients with anaemia undergoing radiotherapy: randomised,
double-blind, placebo-controlled trial. Lancet.
2003;362:1255-1260.
63. Hedenus M, Vansteenkiste J, Kotasek D, Austin
M, Amado RG. Darbepoetin alfa for the treatment
of chemotherapy-induced anemia: disease progression and survival analysis from four randomized, double-blind, placebo-controlled trials. J Clin
Oncol. 2005;23:6941-6948.
From www.bloodjournal.org by guest on March 6, 2016. For personal use only.
2007 109: 868-873
doi:10.1182/blood-2006-08-019083 originally published
online October 10, 2006
New agents that stimulate erythropoiesis
H. Franklin Bunn
Updated information and services can be found at:
http://www.bloodjournal.org/content/109/3/868.full.html
Articles on similar topics can be found in the following Blood collections
Free Research Articles (3658 articles)
Hemostasis, Thrombosis, and Vascular Biology (2494 articles)
Red Cells (1174 articles)
Review Articles (619 articles)
Information about reproducing this article in parts or in its entirety may be found online at:
http://www.bloodjournal.org/site/misc/rights.xhtml#repub_requests
Information about ordering reprints may be found online at:
http://www.bloodjournal.org/site/misc/rights.xhtml#reprints
Information about subscriptions and ASH membership may be found online at:
http://www.bloodjournal.org/site/subscriptions/index.xhtml
Blood (print ISSN 0006-4971, online ISSN 1528-0020), is published weekly by the American Society
of Hematology, 2021 L St, NW, Suite 900, Washington DC 20036.
Copyright 2011 by The American Society of Hematology; all rights reserved.