Electrolysis
advertisement

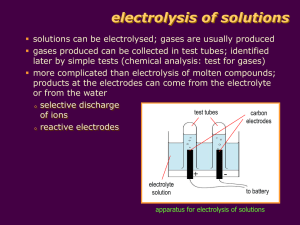
1 Electrolysis Concepts (i) Electrolysis (iii) Types of conductors (ii) Conduction of electricity (iv) Electrolytes and non-electrolytes Introduction Understanding of electrical nature of matter began in the sixteenth century. However, the real break through came only by late 18th century when the Italian physician and anatomist Luigi Galvani marked the birth of electrochemistry by establishing a bridge between chemical reactions and electricity. Volta’s experiments suggested that electricity could be generated by the contact of different metals in moist atmosphere. Nicholson and Charlisle, in 1800, using Volta’s battery decomposed water to produce hydrogen and oxygen. That was, in fact, the first proof to give composition of water. This was electrolysis. It soon became a method of using a direct electric current to drive a chemical reaction which was otherwise non-spontaneous. Electrolysis is the passage of a direct electric current through an ionic substance that is either molten or dissolved in a suitable solvent, resulting in chemical reactions at electrodes and separation of materials. Davy prepared the first elemental sodium by the process of electrolysis of sodium hydroxide melt. Faraday showed that there is a direct relation between the amount of electrical charge passed through the solution and the quantity of the product formed during electrolysis. Two laws of electrolysis are named after him. Today, electrolysis has become a tool in science (and process in industry) for electroplating of metals, electrorefining of metals and production of some chemicals. Requirements of electrolysis Generally, following components are required for electrolysis. (i) An electrolyte - It is a substance containing free ions which are the carriers of electric current in the electrolyte. If the ions are not mobile, as in solid salt, then electrolysis can not occur. For example, an aqueous solution of common salt can act as an electrolyte because it contains free Na+ and Cl- ions which are the carriers of electric current in solution but solid sodium chloride can not act as an electrolyte because it does not have free Na+ and Cl- ions. (ii) A direct current (DC) supply - It provides the electrical energy necessary to create or discharge the ions in the electrolyte. Electrical current is carried by electrons in the external circuit. 2 (iii) Two electrodes - Electrode is an electrical conductor which provides the physical interface between the electrical circuit providing the energy and the electrolyte. For example, graphite rods, platinum plates act as conductors /electrodes. Following figure shows the electrolyte, battery ( power supply) and electrodes ( cathode and anode ) required for electrolysis. Fig.1 – Apparatus required for electrolysis Conduction of electricity Conduction of electricity is the movement of electrically charged particles through a transmission medium i.e. electrical conductor. The movement of charge constitutes an electric current. Electric charge flows from higher potential to lower potential level. This flow of charge may arise as a response to an electric field or as a result of a concentration gradient in carrier density called potential difference. The flow of charge also depends upon the material called conductor. Types of conductors Conductors are materials through which an electrical current can flow easily i.e. with low resistance. This can be a metal or an ionic solution or an ionized gas. Usually the term conductor means current – carrier component of an electrical system through which current can be easily carried. Generally three types of conductors are described. (i) Metallic conductors - In metallic conductors, electrons carry the current and the material is unaffected by this flow ( for small currents ). This type of conductivity is found in solid and liquid ( molten ) metals and In semiconductors current is carried mostly by majority carriers , they may be electrons or holes.. For example, 3 aluminium, copper, are this type of conductors. and silicon is a semiconductor. (ii) Ionic or Electrolytic conductors - In ionic or electrolytic conductors, positively and negatively charged ions ( cations and anions respectively ) carry the electric current. This transport of material changes the composition of the electrolyte and leads to a chemical reaction in the material such as deposit on the electrode. This type of conductivity is found in some solids ( special salts like beta alumina or yttria stabilized zirconia), molten salts and salt solutions. For example, molten sodium chloride, fused cryolite ( Na3AlF6) are this type of conductors. (iii) Plasma conductors – This is a special type of conductor. These are ionized gases. In them, one component is electrons and the other is positively charged gas particles. Both components move in an electric field. For example, gases like argon, neon etc. in discharge tubes act as this type of conductor. Electrolytes and Non-electrolytes Let us see the difference between an electrolyte and a non-electrolyte. Electrolyte - The substance which conducts electricity in its molten state or in the form of its aqueous solution is known as electrolyte. For example, acids, bases and salts are electrolytes. Electrolytes produce ions in solution. These ions are free to move in solution hence solution conducts electricity. Ions can be produced in solution by two types of substances (i) ionic compounds ( like NaOH, KNO3 etc.) that dissolve in water to give ions or (ii) gaseous covalent compounds like HCl or HNO3 which react with water to form ions in solution. Non-electrolyte – The substance which does not conduct electricity in its molten state or in the form of its aqueous solution is known as non-electrolyte. For example, cane sugar, urea, glycerine etc. are non-electrolytes. Non-electrolytes are compounds which do not dissociate at all in solution. These compounds do not provide any ions to carry electrical current. Non-electrolytes are mostly covalent compounds ( exceptions – inorganic strong acids ). For example, many compounds of carbon such as methane CH4, benzene C6H6, ethanol C2H5OH, formaldehyde HCHO are non-electrolytes. Following figures show the difference between electrolytes and non-electrolytes. The bulb in the circuit glows when there is an electrolyte like aqueous solution of KNO3 while the bulb does not glow when there is a non- electrolyte like benzene. 4 Electrolyte conducts current in a solution Non electrolyte does not conduct current in a solution Fig. 2 - Difference between electrolyte and non- electrolyte Strong electrolyte - The electrolyte which dissociates or ionizes almost completely to form free mobile ions in the solution or in molten form is called a strong electrolyte. The more the availability of free mobile ions in an electrolyte, the greater is its capacity to carry or conduct current i.e. the stronger the electrolyte. All water soluble salts are strong electrolytes. The strong acids like HCl, H2SO4, HNO3, strong bases like NaOH, KOH are strong electrolytes. Weak electrolyte - The electrolyte which ionizes or dissociates only partially to form a few free mobile ions in molten state or in solution is called a weak electrolyte. . Most of the weak electrolyte remains as unionized molecules. When the number of mobile ions are less in the electrolyte, lesser is the capacity to carry or conduct the electric current i.e. the weaker is the electrolyte. For example, in acetic acid, the number of ions ( acetate and hydrogen ions ) is less compared to the total amount of acetic acid molecules present. So acetic acid is a weak electrolyte. Similarly, ammonium hydroxide is a weak electrolyte. Following figure shows the difference between a strong electrolyte and a weak electrolyte. The bulb in the circuit glows strongly when there is a strong electrolyte like aqueous solution of common salt while the bulb in the circuit glows weakly when there is a weak electrolyte like acetic acid. 5 Fig.3 – Difference between strong electrolyte and weak electrolyte Activity 1 – Take two medium size porcelain dishes. Take four beakers of 250 ml capacity. Put two graphite rods in each porcelain dish and in each beaker. Connect the two rods to a battery ( a source of electric current ) by means of wires. Introduce a bulb in the circuit. (i) In the first porcelain dish, take about 10 to 15 grams of sodium chloride. Place the two graphite rods in it. Pass electric current ( apply the potential difference . using power supply ) through the solid sodium chloride. The bulb does not glow which means electric current does not pass through it. This shows that solid sodium chloride is not an electrolyte and is not a conductor of electricity. (ii) In the second porcelain dish, take 10 to 15 grams of sodium chloride. Heat the dish till the solid melts. Place the two graphite rods in the molten sodium chloride. ( Apply the potential difference using power supply ) Pass electric current through the molten sodium chloride. The bulb glows which means electric current passes through it. This shows that molten sodium chloride is an electrolyte and is a conductor of electricity. (iii) In the first beaker, take about 10 to 15 grams sodium chloride and 100 ml water. Stir the solution. Place the two graphite rods in it. ( Apply the potential difference using power supply ) Pass electric current through the solution. The bulb glows which means electric current passes through it. This shows that aqueous solution of sodium chloride is an electrolyte and it is a conductor of electricity. (iv) In the second beaker, take about 10 to 15 grams copper sulphate and 100 ml water. Stir the solution. Place the two graphite rods in it. ( Apply the potential difference using power supply ) Pass electric current through the solution. The bulb glows which means electric current passes through it. This shows that aqueous solution of copper sulphate is an electrolyte and it is a conductor of electricity (v) In the third beaker, take about 10 to 15 grams urea and 100 ml water. Stir the solution. Place the two graphite rods in it. ( Apply the potential difference using power supply ) Pass electric current through the solution. The bulb does not glow which means electric current does not pass through it. This shows that aqueous solution of urea is not an electrolyte and is not a conductor of electricity. 6 (vi) In the fourth beaker, take about 10 to 15 grams glucose and 100 ml water. Stir the solution. Place the two graphite rods in it. ( Apply the potential difference using Power supply to ) . Pass electric current through the solution. The bulb does not glow which means electric current does not pass through it. This shows that aqueous solution of glucose is not an electrolyte and is not a conductor of electricity. This experiment shows that solid sodium chloride, aqueous solutions of urea and glucose are non-electrolytes because they do not allow the passage of electric current through them. On the other hand, molten sodium chloride, aqueous solutions of sodium chloride and copper sulphate are electrolytes because they allow the passage of electric current through them. Activity 2- Let us try to understand the difference between a strong electrolyte and a weak electrolyte. This activity should be done under the supervision of teacher. Take about 50 ml of 0.01 N KCl solution in a conductivity cell. Measure its conductance with a meter as shown in the diagram Fig.4 – Measurement of conductance of an electrolyte Wash the conductivity cell and the beaker with distilled water. Now take 50 ml of 0.01 N acetic acid solution in the conductivity cell. Measure its conductance with a meter in a similar manner. You will find that the conductance of KCl solution is much more than that of acetic acid solution. This shows that KCl is a strong electrolyte while acetic acid is a weak electrolyte. Check your understanding (i) Find out the type of conductor from the following: Fused cryolite , argon gas, mercury, solution of NaCl, zinc plate (ii) Which of the following are electrolytes and which are non-electrolytes ? Ice, molten lead bromide, neon gas, aqueous solution of zinc chloride, Sulphur, Air (iii) Which of the following are strong electrolytes and which are weak electrolytes ? Aqueous solution of benzoic acid, Nitric acid, Citric acid, Aqueous solution of ammonia, Solution of sodium carbonate, Molten potassium hydroxide 7 Concepts (i) Electrolytic cell (ii) Mechanism of electrolysis (iii) Factors affecting preferential discharge of ions during electrolysis Electrolysis is carried out in an electrolytic cell. Electrolytic cell - It is a devise which contains two electrodes ( cathode and anode ) in contact with an electrolyte and that brings about a chemical reaction when connected to an outside source of electricity. It is also known as a voltameter. Following figure shows an electrolytic cell. In this cell, applied voltage causes a reaction to occur which is otherwise non-spontaneous. For example, breakdown of water into hydrogen and oxygen is such a non-spontaneous reaction. In the electrolytic cell, the electrodes are made of some metal (or sometimes graphite) and when connected to direct current, one electrode becomes positively charged and the other becomes negatively charged. This initiates the movement of ions in the electrolyte towards the electrodes. Positive ions move towards the negative electrode ( cathode ) and negative ions move towards the positive electrode ( anode ). Then a chemical reaction takes place at each electrode with ions changing from positive or negative to neutral atoms or molecules. Fig. 5 - Electrolytic cell Convention about electric current - As a convention, the positive terminal of the battery is taken to be at a higher electrical potential than the negative terminal. Hence conventional direction of electric current in a circuit is from a positive terminal to a negative terminal of battery. However, electrons, which are negatively charged, flow from the negative terminal to the positive terminal of the battery. During electrolysis, electrons leave the electrolyte at the anode and electrons enter the electrolyte at the cathode. 8 Mechanism of electrolysis - Michael Faraday showed that products of electrolysis appear only at the surfaces of electrodes. This fact led Swante Arrhenius in 1887 to forward his hypothesis of ions in order to explain the phenomenon of electrolysis. According to this hypothesis, the molecules of the electrolyte, when in the state of solution or in the fused or molten state, break up into electrically charged particles known as ions which under the influence of the electric current travel towards the electrodes. Those traveling towards the cathode are called cations ( or positive ions ) while those traveling towards the anode are called anions ( or negative ions ). The velocity with which these ions move may or may not be equal. When the anions are actually discharged at the surface of the anode and the cations similarly at the cathode, primary reactions ( electrode processes ) take place. In the primary reaction at the cathode, ( cathodic process ), electrons are taken by the electrolyte from the cathode and in the primary reaction at the anode ( anode process ), electrons are given up by the electrolyte to the anode. Thus an electric current which passes through the electrolyte consists of a flow of electrons, indicated by the overall transfer of electrons from the cathode to the anode in the external circuit. Illustration - Let us consider the example of sodium chloride NaCl . We know that NaCl does not conduct electricity as it is. Sodium chloride is an ionic compound and both the Na+ and the Cl- ions are strongly attracted to each other by electrostatic attraction in solid state. The strength of the electrical current ( field ) is unable to break the ionic bond. Not only breaking of the ionic bond is needed, the flow of charges also has to take place. This does not happen in solid NaCl. Thus solid NaCl is not an electrolyte. Fig. 6 – Cations and anions are held together strongly On the other hand, in case of molten NaCl, the bond between the Na+ and the Clions has loosened. The bond is weakened. Hence the ions can become mobile and conduct electricity. Fig. 7 – Cations and anions held together loosely 9 In an aqueous solution of NaCl, water molecules separate the Na+ and the Cl- ions. This makes them very mobile. The mobility is enhanced when two electrodes in the form of anode (+) and cathode (-) are inserted in the salt solution. Water molecules also partially dissociate into H+ and OH- ions. The Na+ and H+ ions get attracted toward the cathode and the Cl- and OH- ions get attracted toward the anode. The aqueous solution of NaCl is therefore a good electrolyte. The product of electrolysis are H2 at the cathode and Cl2 at the anode. Fig. 8 – Cations and anions are separated in a solution We can conclude from the above discussions that the movement of ions is responsible for the flow of current in an electrolytic cell. Electrolysis of molten sodium chloride - Reactions at the electrodes 1. Dissociation of NaCl : 2. Reaction at the cathode : Reaction at the anode : Na + Cation NaCl Na + + 1e- ClCl Na 1e- + Cl + Cl + ClAnion (neutralization) (neutralization) Cl2 Overall chemical reaction - 2 NaCl → 2 Na + Cl2 Following figures show the electrolysis of solid sodium chloride, molten sodium chloride and aqueous solution of sodium chloride. 10 Fig. 9 – Electrolysis of solid NaCl Fig. 10 – Electrolysis of molten NaCl Fig. 11 – Electrolysis of aqueous NaCl 11 Factors affecting preferential discharge of ions during electrolysis When the electrolyte is an aqueous solution, the electrolytic reactions become more complicated. Water comes in the picture as water ionizes to give few H+ and OH— ions. The cations and anions compete with H+ and OH- ions respectively for the discharge. All the cations migrate to the cathode and all the anions migrate to the anode but all of them are not discharged at the electrodes. Only one cation and only one anion is selectively discharged at the electrode. Following factors determine the preferential discharge of the ion at the electrode. (i) The position of the ion in the electrochemical series - Electrochemical series is an arrangement of cations and anions in the order of their decreasing reactivity. Usually standard oxidation or reduction potential is taken as the base for determining the reactivity of the ion. Following series shows the electrochemical series for cations and anions in the decreasing order of reactivity. Cations – K+> Na+ >Ca2+ > Mg2+ > Al3+ >Zn 2+ >Fe2+ > Sn2+ > Pb2+ >H+ > Cu2+ > Ag+ Anions - SO42 - > NO3- > CO32 - > OH- > Cl - > Br - > S2- > I As a rule, the cation or anion which is lower in position in the electrochemical series is discharged first. Further, the anion which does not contain oxygen, is discharged in preference to that which contains oxygen. For example, when dilute aqueous solution of potassium chloride is subjected to electrolysis, H+ ions are discharged at the cathode in preference to K+ ions because H is below K in the electrochemical series. Similarly, Cl- ions are discharged at the anode in preference to OH- ions because Cl is below OH in the electrochemical series. The products of electrolysis are thus H2 (and not K )at the cathode and Cl2 ( and not O2 ) at the anode. (ii) Concentration of the ion in the electrolyte – If the concentration of a particular ion is high, it is discharged first even though it is higher in the electrochemical series compared with another ion present in the solution. . For example, if dilute solution of sodium chloride is electrolyzed, hydrogen gas is given off at the cathode . However, when concentrated solution of sodium chloride is electrolyzed, Na+ ions are discharged at the cathode in preference to H+ ions because the concentration of Na+ ions is much higher than that of H+ ions. (iii) Nature of the electrode – Electrodes used in the process of electrolysis are either active or inert. The electrodes used in electrolysis determine the ion preferred for discharge. If inert electrodes like platinum or graphite are used, they will not take part in the electrolytic reaction. The electrolysis will then depend upon only the above two factors i.e. position of ion in the electrochemical series and the concentration of the ion. The active electrodes like Cu, Ni, Ag, if used, take part in the electrolytic reaction and the product formed is different. For example, in the electrolysis of aqueous solution of CuSO4, if copper electrodes are used, copper 12 atoms are deposited at the cathode and copper ions are formed at the anode. But if platinum electrodes are used, copper atoms are deposited on the cathode and oxygen gas is evolved at the anode. Thus the products formed during electrolysis are different for different electrodes. Activity 3 - Carry out the following experiment under the supervision of your teacher. This experiment illustrates the phenomenon of electrolysis and the preferential discharge of ions in electrolysis, based on their position in the electrochemical series. Take 150 ml. of 0.1 M copper sulphate solution in a 250 ml beaker. Place two graphite electrodes in it as shown in the figure. Connect the two electrodes to a 6 volt battery. Fig. 12 - Electrolysis of 0.1 M solution of copper sulphate In aqueous solution, copper sulphate gives Cu2+ ions and SO42- ions and water gives H+ and OH- ions. Thus, there are two cations H+ and Cu2+ and two anions OH- and SO4 2- . There will be a question of preferential discharge of the ion at the electrode. We refer to the electrochemical series of cations and anions and make our predictions. When the electrolysis starts, Cu2+ ions being lower in position in the electrochemical series, are discharged at the cathode in preference to H+ ions. Similarly, OH- ions being lower in position are discharged at anode in preference to SO42- ions. As a result, copper is deposited on the cathode and oxygen gas is evolved at the anode. The electrode reactions are as follows. At cathode Cu2+ + 2e- → Cu At anode 4OH → O2 + 2 H2O + 4eWe see a reddish brown deposit on one of the electrodes ( cathode) which confirms the deposit of copper. If a glowing splinter is taken near the anode, it burns more vigorously. This confirms the evolution of oxygen gas at the anode. 13 Activity 4 – Carry out the following experiment under the supervision of your teacher. This experiment illustrates the preferential discharge of ions in electrolysis according to the concentrations of those ions. In a 250 ml beaker, take 100 ml of almost saturated solution of sodium chloride. This is taken to be highly concentrated solution of sodium chloride. Place two graphite electrodes in it. Connect a 6 volt battery to the two electrodes. In the electrolyte, there are Na+ and H+ ions present as cations and OH- and Clions are present as anions. Since two cations and two anions are present, question of preferential discharge will arise. When the electrolysis starts, since concentration of Na+ ions is very large as compared to that of H+ ions ( the degree of dissociation of water is very low which is 10-7 per mol hence the concentration of H+ ions is very low ), Na+ ions are discharged at the cathode in preference to H+ ions though Na+ ion is higher in position than H+ ion in the electrochemical series. Similarly, concentration of Cl -being very large as compared to OH- ions, Cl- ions are discharged at anode in preference to OH- ions. Here, by chance, OH- ion is higher in position than Cl- in the electrochemical series. Thus due to high concentration of ions, they ( Na+ and Cl- ) are discharged first irrespective of their position in the electrochemical series. Thus, sodium metal and chlorine gas are the products of electrolysis. Check your understanding (i) Can we call electrolysis as a ‘ redox’ reaction ’? (ii) Arrange the following ions in the preferential order of discharge at the electrode during electrolysis ? Pb2+, H +, Au3+, Zn2+ , K+ and SO42- , NO3 - , OH-, Cl -, Br(iii) Why an ionic compound is a bad conductor of electricity in solid state while it is a good conductor in molten and aqueous state ? (iv) What will be the products if an aqueous solution of ZnSO4 is subjected to electrolysis using graphite electrodes ? 14 Concepts (i) Faraday’s first law of electrolysis (ii) Faraday’s second law of electrolysis. The quantitative aspect of experimental electrolysis was summarized by Faraday in the year 1834 in two statements which later came to be known as Faraday’s laws of electrolysis. In order to understand Faraday’s laws, it is necessary to refer to terms like ampere, coulomb and Faraday. Coulomb is the quantity of electricity that passes through the circuit when a current of one ampere flows for one second. Faraday is the quantity of electricity required to liberate one gram equivalent of silver or any other element during electrolysis. Ampere is the current which when passes through a circuit for one second liberates 0.0000104 g of hydrogen or 0.001118 g of silver. First law - The weight of a substance liberated at an electrode during electrolysis is directly proportional to the quantity of electricity passed through the electrolyte. If W is the weight of the substance liberated and Q is the quantity of electricity, then W α Q Further, Q = I x t where, I is the current in amperes and t is the time in seconds. Thus, W α I x t or W = Z I t where Z is a constant called Electro Chemical Equivalent ( ECE). The electro chemical equivalent Z is defined as the weight of the substance liberated or deposited during electrolysis when the quantity of electricity passing through the electrolyte is one coulomb. It is given by the formula Eq.Wt. of a substance / 96500. Second law - When the same quantity of electricity is passed through different electrolytes, the weights of the substances liberated at the respective electrodes are directly proportional to the chemical equivalents ( equivalent weights ) of substances. For example, if same quantity of electricity is passed through solutions of copper sulphate and silver nitrate, then the quantity of copper and silver deposited on respective electrodes are in proportion to their equivalent weights. Weight of copper deposited -------------------------------Weight of silver deposited = C.E. of copper E.C.E. of copper ------------------- = -----------------------C. E. of silver. E.C.E. of silver 15 Activity 5 – Carry out the following experiment under the supervision of your teacher. This is quantitative electrolysis of aqueous copper (II) sulphate. This is verification of Faraday’s first law of electrolysis. Set up the circuit as shown in the diagram.. Use crocodile clips to hold the electrodes. Make sure that the electrodes do not touch each other. Fig. 13 – Electrolysis of copper sulphate solution using copper electrodes Take about 100 ml of 0.1M aqueous solution of copper sulphate in the beaker. Switch on the current, set the rheostat so that a current of 0.5 amperes passes through the solution. Switch off the current. Clean the above two copper electrodes with emery paper, wash them with water and dry them by hot air blower. Mark the electrodes as + and – at one end and weigh them separately. Record their weights. Start the current and stop watch simultaneously. (It is difficult to avoid fluctuations in current throughout the electrolysis and thus obtain an accurate value to use in the calculations.). After 30 minutes switch off the current and stop the clock. Remove the electrodes from the electrolyte, dip them in little organic solvent propanone to wash, take them out and allow them to air dry. Reweigh the dry electrodes. You will find that the increase in weight of cathode ( weight of copper deposited ) is approximately equal to the decrease in weight of anode ( loss in weight of anode ). Further we can verify Faraday’s first law of electrolysis by using its mathematical relation W = Z I t , where W is the weight of copper deposited on the electrode, Z is electrochemical equivalent (E.C.E.) which is a constant , I is the current in amperes and t is the time in seconds for which current is passed. In the present experiment, Z = 31.75 / 96500 ( the equivalent weight of copper is 63.5 / 2 = 31.75 ) , I = 0.5 A and t = 30 x 60 = 1800 seconds. Hence W = 31.75 x 0.5 x 1800 / 96500 = 0.2962 g 16 We can compare this weight with the actual weight of copper deposited on the electrode during electrolysis. If the two weights match, we can say that Faraday’s first law stands verified. ( Due to current fluctuations, the weight obtained experimentally may be little different than that obtained theoretically.) Check your understanding (i) What mass of zinc will be deposited if a current of 0.22 amperes flows through the cell for 1.5 hours ? ( At.. wt. of zinc = 65 ) (ii) Exactly 0.4 faraday electric charge is passed through three electrolytic cells in series, first containing AgNO3, second CuSO4 and third FeCl3 solution. How many gram of each metal will be deposited assuming only cathodic reaction in each cell? (iii) What is the relation between chemical equivalent weight and electrochemical equivalent ? 17 Concepts Applications of Electrolysis (i) Electroplating (iii) Extraction of metals from their ores (ii) Electro-refining of metals (iv) Anodizing 1) Electroplating Electroplating is an electrolytic process by which a thin and compact layer of a less reactive or more noble metal is deposited on article made of a more reactive metal. The process of electroplating involves following steps. (i) Before electrolysis, the metal surface is cleaned thoroughly. Firstly, an alkaline solution is used to remove grease and then it is treated with acid to remove any oxide layer. It is then washed with water. (ii) The article to be electroplated is made cathode since metallic ions are positive and thus get deposited on the cathode. (iii) The anode is made of pure metal which is to be coated on the article. (iv) The electrolyte is the salt of the metal to be coated on the article. (v) A direct current ( D.C.) is passed through the electrolyte. The anode dissolves depositing the metal ions from the solution on the article in the form of a metallic coating. The passage of low current is continued for a long time to ensure an even coating. (vi) To obtain a thin, coherent and bright deposit, the conditions of low current density, optimum temperature and low metal ion concentration are found to be helpful. The choice of electrolyte for use in the electroplating bath is very important. A good electrolyte should have following properties. (i) (ii) (iii) (iv) It should be highly soluble in water. Its solution should be a reasonably good conductor of electricity. Its solution should be stable towards oxidation, reduction and hydrolysis. It should be reasonably priced. 18 Let us see the electroplating of nickel. It is done with following conditions. Electrolyte – Aqueous solution of nickel sulphate; Cathode – Article to be electroplated ( say nail ) Anode – Plate of pure nickel Following figure shows electroplating with nickel. Fig.14 - Electroplating with nickel Reactions during electrolysis – Dissociation of the electrolyte - NiSO4 → Ni2+ + SO42Reaction at the cathode - Ni2+ + 2e- → Ni ( Preferential discharge of Ni2+ takes place ) Reaction at the anode – Ni → Ni2+ + 2e- ( OH- and SO42- are not discharged.) Electroplating offers following advantages – (i) It offers surface protection to a metal e.g. nickel plating on iron protects it from corrosion. (ii) It makes the article attractive. For example, electroplating of gold or silver on brass makes its look beautiful. Activity 6 – Carry out this experiment either individually or in a small group under the supervision of your teacher. This is an experiment of electroplating of silver on a copper spoon ( or any other small copper article. ) Clean a copper spoon with emery paper and dip it in a dilute solution of aqua regia to clean it further. . Dry the spoon by wiping with cotton. Take 3 g each of NaOH, AgCN and NaCN and dissolve this mixture in 100 ml of water. Alternatively, to a dilute solution of silver cyanide add sodium cyanide solution till the precipitate first formed just dissolves. This is a dilute solution of sodium argento cyanide Na [ Ag (CN)2] which serves as the electrolyte. Take this electrolyte in a small metal tank or a trough. Attach the copper spoon to a copper wire and attach the copper wire through a crocodile clip to the negative terminal of the 9 volt battery so that the copper spoon behaves as a cathode. Take a pure silver plate and attach it to the positive terminal of the battery so that the silver plate behaves as anode. Place the two electrodes in the electrolyte. Switch on the 19 current. Electrolysis starts. To obtain a thin, coherent and a bright deposit of silver , a direct current with low current density and low metal ion concentration are essential. So a current of about 0.5 A is passed for one hour. Fig. 15 – Electroplating with silver The anode dissolves depositing the silver ions from the solution on the spoon in the form of a metallic coating. The reactions that take place during electrolysis are as follows. Dissociation of the electrolyte At Cathode At Anode - Na[Ag(CN)2] → Na+ + Ag+ + 2 CNAg+(aq) + e- → Ag (s) Ag (s) → Ag+(aq) + e- Thus a less active metal , silver is deposited on a more active metal, copper by the process of electroplating. 2) Electro – refining of metals The process by which impurities in a metal are removed electrolytically to obtain a highly pure metal is called electro-refining. The process of electro-refining of metals involves following steps (i) The electrolyte is usually an aqueous solution of the salt of the metal with some corresponding acid, if necessary. (ii) A thick plate of the impure metal is made the anode. (iii) A thin rod or sheet of pure metal is made the cathode. (iv) The metal ions being positive migrate towards the cathode and get discharged. (v) At anode, the atoms of the metal lose electrons to form cations and enter the solution. (vi) The less electropositive impurities in the anode, settle down at the bottom and are removed as anode mud while the more electropositive impurities pass into the 20 solution. Let us see the electro-refining of copper. It is done with following conditions. Electrolyte – Aqueous solution of copper sulphate; ( acidified ) Cathode – Pure copper metal ( thin rod ) Anode – Impure copper metal ( thick sheet ) Following figure shows electro-refining of copper metal. Fig.16 – Purification of impure copper Reactions during electro-refining – Dissociation of the electrolyte - CuSO4 → Cu2+ + SO42Reaction at the cathode - Cu2+ + 2e- → Cu ( Preferential discharge of Cu2+ takes place ) Reaction at the anode – Cu → Cu2+ + 2e- ( OH- and SO42- are not discharged.) Electro-refining offers following advantages – (i) Metals like nickel, zinc, lead and copper are refined by this method. (ii) This method sometimes yields valuable metals in the form of anode mud e.g. silver and gold are collected in the anode mud during the electro-refining of copper. 3) Extraction of metals from their ores In this process, the ore is first treated by some chemical method and then brought to its chloride or oxide form. It is then melted and subjected to electrolysis to obtain the metal. Highly electropositive metals like potassium , calcium, sodium , aluminium are extracted by electrolysis of their fused chlorides or oxides. For example, sodium is obtained from molten sodium chloride, aluminium is obtained from molten aluminium oxide (with cryolite) by electrolysis after giving a chemical treatment to their ores. 21 4) Anodizing Anodizing, or anodising in British English, is an electrolytic passivation process used to increase the thickness of the natural oxide layer on the surface of metal parts. The process is called "anodizing" because the part to be treated forms the anode electrode of an electrical circuit. Anodizing increases corrosion resistance and wear resistance and provides better adhesion for paint primers and glues than bare metal. Anodic films can also be used for a number of cosmetic effects, either with thick porous coatings that can absorb dyes or with thin transparent coatings that add interference effects to reflected light. Anodic films are most commonly applied to protect aluminium alloys, although processes also exist for titanium, zinc, magnesium, niobium, and tantalum. This process is not a useful treatment for iron or carbon steel because these metals exfoliate when oxidized; i.e. the iron oxide (also known as rust) flakes off, constantly exposing the underlying metal to corrosion. Aluminium alloys are anodized to increase corrosion resistance, to increase surface hardness and to allow dyeing (colouring), improved lubrication or improved adhesion. The anodic layer is non-conductive. Anodizing of aluminium Many metals are structurally weakened by the oxidation process but not aluminium. Aluminium becomes stronger and more durable through the process of anodizing. For this, sheet of aluminium is placed in an acid bath ( 5M sulphuric acid.). The sheet of aluminium becomes an anode and the tank containing the electrolyte becomes a cathode. An electric current is passed through the electrolyte. Hydrogen gas is evolved at the cathode and oxygen gas is evolved at the anode. It causes the surface of aluminium to oxidise ( essentially rust ). The oxygen combines with the aluminium to form aluminium oxide. The oxidised aluminium ( black Al2O3 ) forms a strong coating as it replaces the original aluminium on the surface. This is called anodizing of aluminium. The experimental set up of anodizing is shown below. Fig. 17 - Anodizing of aluminium 22 The coating has ability to absorb dyes into the microscopic porosity of its surface. Hence usually organic dyes are added to impart colour to the surface of the article which makes the look of the article attractive. Anodizing finds applications in making gift articles, kitchen wares, name plates, decorative pieces etc. Check your understanding (i) Suggest the equipment and set up required for electroplating of zinc on iron. (ii) Theoretically any metal can be anodized but anodizing of aluminium is more common. Why ? (iii) Which metals are obtained from their ores by electrolytic method ? 23 References / Diagrams / Figures – 1) Fig.1 - Apparatus required for electrolysis www.rustyiron.com/engines/electrolysis/index.html 2) Fig. 2 - Difference between electrolytes and non-electrolytes http://www.tutorvista.com/content/chemistry/chemistry-ii/electrolysis/electrolytes-and -nonelectrolytes.php 3) Fig. 3 - Difference between strong electrolyte and weak electrolyte http://www.tutorvista.com/content/chemistry/chemistry-ii/electrolysis/electrolytesand –nonelectrolytes.php 4) Fig. 4 - Measurement of conductance of an electrolyte 5) Fig. 5 -Electrolytic cell www.rustyiron.com/engines/electrolysis/index.htm 6) Fig. 6 - Cations and anions are held together strongly www.educationalelectronicsusa.com/c/electrolysis-1.htm 7) Fig. 7 - Cations and anions are held together loosely www.educationalelectronicsusa.com/c/electrolysis-1.htm 8) Fig. 8 - Cations and anions are separated in solution www.educationalelectronicsusa.com/c/electrolysis-1.htm 9) Fig. 9 - Electrolysis of solid NaCl www.educationalelectronicsusa.com/c/electrolysis-1.htm 10) Electrolysis of molten NaCl www.educationalelectronicsusa.com/c/electrolysis-1.htm 11) Electrolysis of aqueous NaCl www.educationalelectronicsusa.com/c/electrolysis-1.htm 12) Electrolysis of 0.1 M solution of copper sulphate http://www.practicalchemistry.org/experiments/electrolysis-of-copperiisulphatesolution,108,ex.html 13) Electrolysis of copper sulphate solution using copper electrodes http://www.tutorvista.com/content/physics/physics-iv/thermal-chemicalcurrents/chemical-effects-current.php 24 14) Electroplating with nickel http://www.tutorvista.com/content/chemistry-ii/electrolysis/electroplating.php 15) Electroplating with silver http://www.tutorvista.com/content/chemistry-ii/electrolysis/electroplating.php 16) Purification of impure copper http://image.tutorvista.com/content/electrolysis/copper-purification.gif 17) Anodizing of aluminium Anodising- Images http://www.practicalchemistry.org/data/images/width400/anodising-aluminium47,jpg